The aim of the proposed recommendations is to be a tool to facilitate decision-making in patients with a solitary pulmonary nodule (SPN). For an optimal decision, accessibility to the different diagnostics techniques and patient preferences need to be incorporated.
The first assessment, which includes a chest computed tomography scan, separates a group of patients with extrapulmonary neoplasm or a high surgical risk who require individualized management. Another two groups of patients are patients with SPN up to 8mm and those who have a subsolid SPN, for which specific recommendations are established.
SPNs larger than 8mm are classified according to their probability of malignancy into low (less than 5%), where observation is recommended, high (higher than 65%), which are managed with a presumptive diagnosis of localized stage carcinoma, and intermediate, where positron emission tomography-computed tomography has high yield for reclassifying them into high or low probability. In cases of intermediate or high probability of malignancy, transbronchial needle aspiration or biopsy of the nodule may be an option.
Radiologic observation with low radiation computed tomography without contrast is recommended in SPN with low probability of malignancy, and resection with videothoracoscopy in undiagnosed cases with intermediate or high probability of malignancy.
Las recomendaciones que se proponen pretenden ser un instrumento que facilite la toma de decisiones en pacientes con nódulo pulmonar solitario (NPS). Para una decisión óptima hay que incorporar la accesibilidad a las distintas técnicas diagnósticas y las preferencias del paciente.
La primera valoración, que incluye la tomografía computarizada torácica, separa a un grupo de pacientes con neoplasia extrapulmonar o muy alto riesgo quirúrgico que requieren manejo individualizado. Otros 2 grupos son los pacientes con NPS de hasta 8mm y los que tienen NPS subsólido, para los que se establecen recomendaciones específicas.
Los NPS mayores de 8mm se clasifican según su probabilidad de malignidad en baja (menor del 5%) donde se recomienda observación, alta (mayor del 65%) que se manejan con el diagnóstico de presunción de carcinoma en estadio localizado, e intermedia, donde la tomografía de emisión de positrones tiene gran rendimiento para reclasificarlos en alta o baja probabilidad. En los casos de probabilidad de malignidad intermedia o alta puede ser una opción la punción o biopsia transbronquial del nódulo.
Se recomienda la observación radiológica con tomografía computarizada de baja radiación y sin contraste en el NPS con baja probabilidad de malignidad, y la resección con videotoracoscopia en los casos no diagnosticados y con probabilidad de malignidad intermedia o alta.
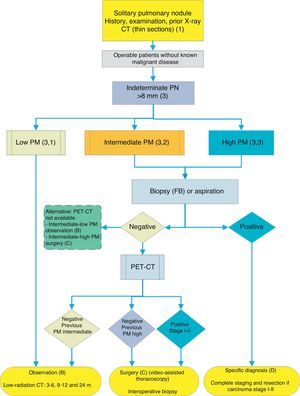
The aim of these guidelines is to facilitate decision making in the treatment of patients with a solitary pulmonary nodule (SPN).1–3 These guidelines are not intended to be rigid, since the treatment of SPN is an example of how estimation of the probability of malignancy (PM), access to the various diagnostic and therapeutic techniques and patient preferences work together to mold the optimal decision. This process should be individualized in the clinical setting and for each particular situation. Since accessibility to some diagnostic techniques may vary, depending on the setting, a general strategic algorithm is proposed (Fig. 1) with two alternatives, depending on the degree of accessibility to positron emission tomography (PET) (Figs. 2 and 3). The recommendations have been graded according to strength (strong 1, weak 2) based on the relationship between the foreseeable benefits and the risks for the patient; and the quality of the scientific evidence, as high (A), moderate (B), low (C) or very low (D), according the GRADE system.4 A summary of these recommendations2,4,6 is given in Table 1. The extended version of these recommendations is available as an online supplement, along with additional tables and figures (S) (Appendix 1).
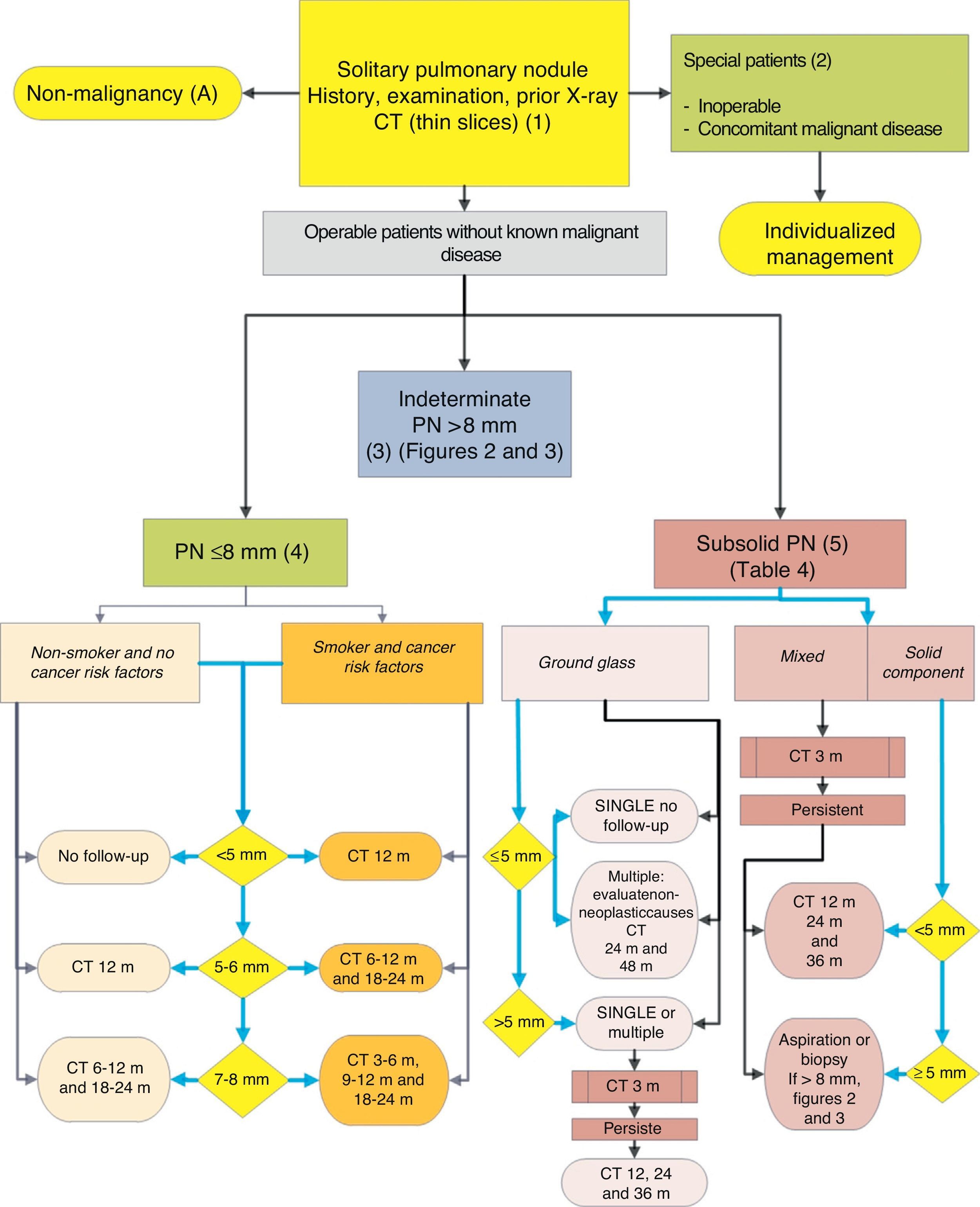
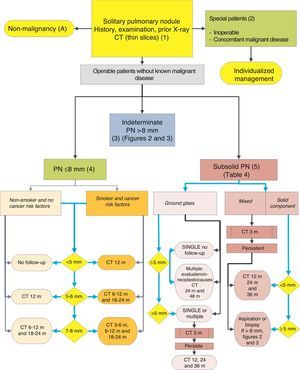
Algorithm for the management of PN: initial classification and observation of SPN≤8mm and subsolid SPN. m: months, PN: pulmonary nodule; SPN: solitary pulmonary nodule; CT: computed tomography, including thin sections. Follow-up with low-radiation CT, provided no growth is detected. Numbers and letters in brackets refer to the sections in the text where they are discussed.
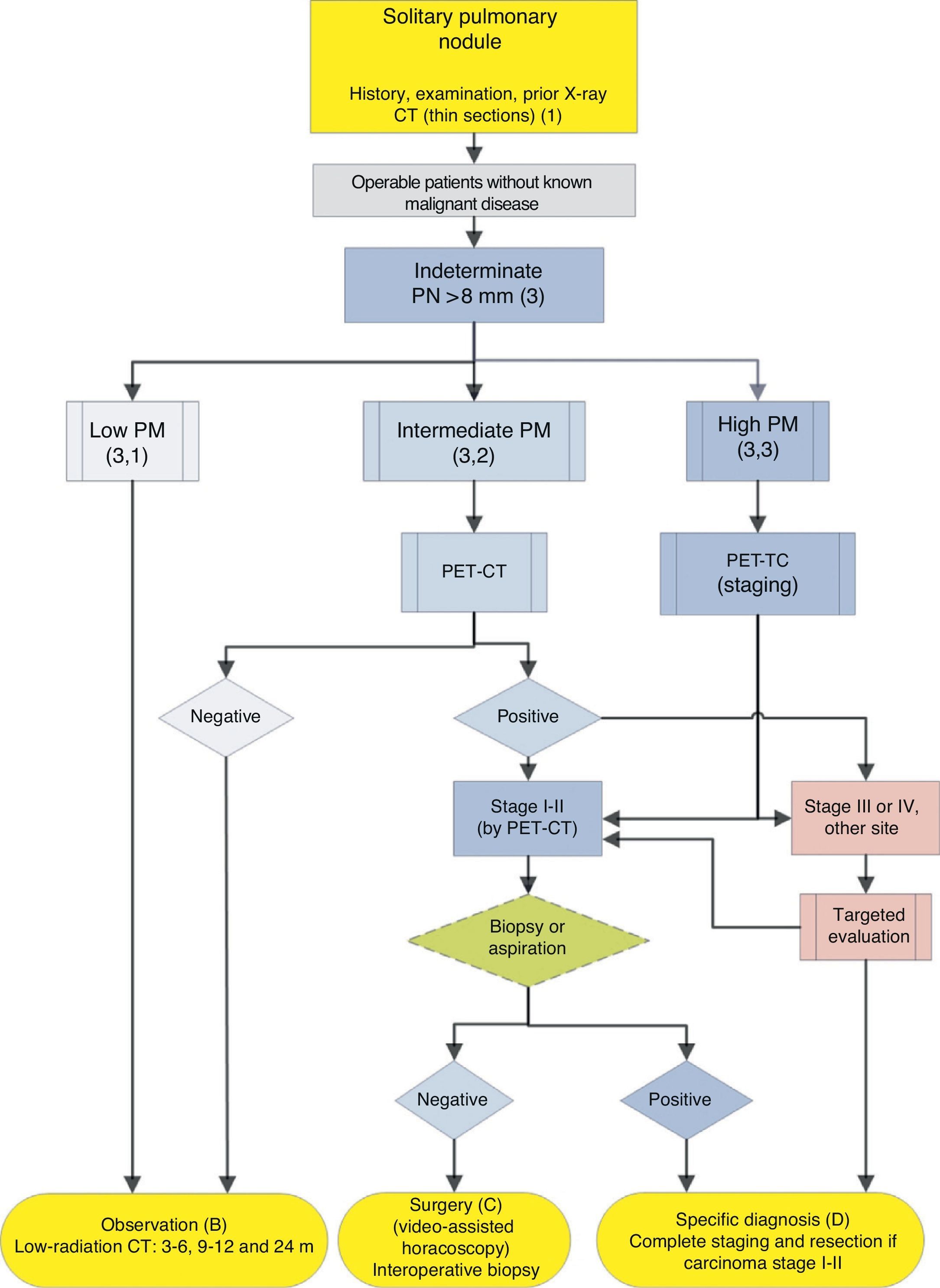
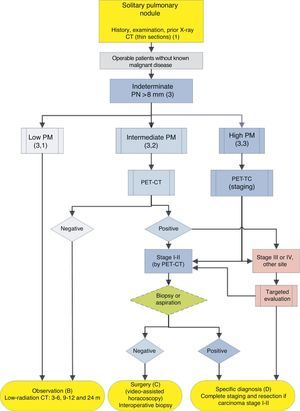
Algorithm for the management of PN>8mm: clinical setting with easy access to PET-CT. m: months, PN: pulmonary nodule; SPN: solitary pulmonary nodule; PET-CT: positron emission tomography with computed tomography; PM: probability of malignancy; CT: chest computed tomography, including thin sections. Follow-up with low-radiation CT, provided no growth is detected. Numbers and letters in brackets refer to the sections in the text where they are discussed.
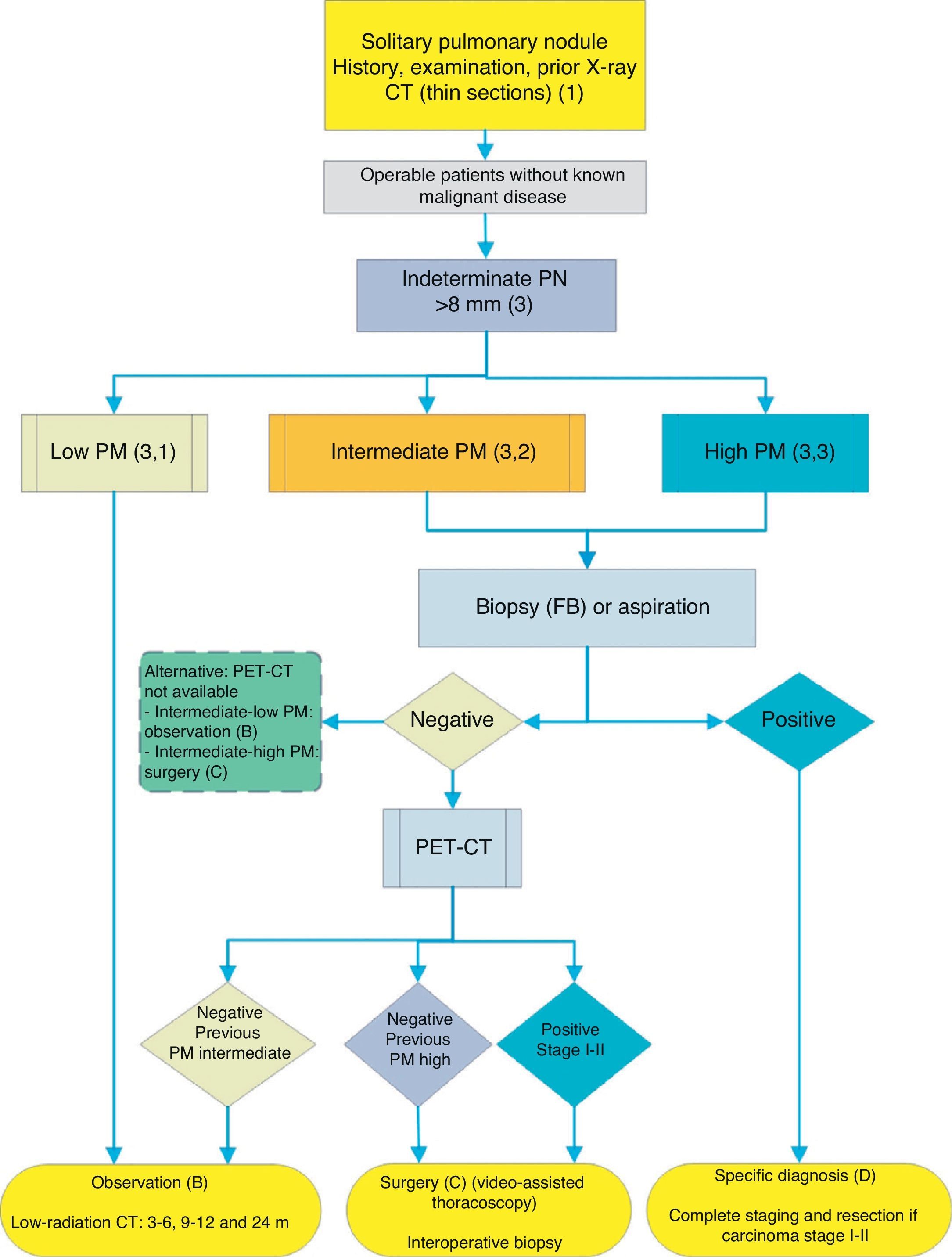
Algorithm for the management of PN>8mm: clinical setting with difficult access to PET-CT or preference for cytohistological study of SPN. FB: fiberoptic bronchoscopy and guided biopsy; m: months; SPN: solitary pulmonary nodule; PET-CT: positron emission tomography with computed tomography; PM: probability of malignancy; CT: chest computed tomography, including thin sections. Follow-up with low-radiation CT, provided no growth is detected. Numbers and letters in brackets refer to the sections in the text where they are discussed.
Recommendations for the Management of Solitary Pulmonary Nodule.
| Recommendation | Gradea |
| SPN initial evaluation | |
| All decisions on the management of an SPN must include the opinion and preferences of the appropriately informed patient | 1C |
| Evaluate stability or growth in previous radiological studies if available | 1C |
| Stability for more than 2 years in solid SPN and benign calcification indicate benignancy and do not require further evaluation | 2C |
| CT, with thin sections through lesion of interest, is essential for the initial evaluation of indeterminate SPN | 1B |
| SPN in patients with previous or concomitant malignancy require individualized management and evaluation | 1C |
| SPNs in inoperable patients require individualized management | 1C |
| SPNs will be classified as solid nodules>8 mm, solid nodules≤8mm and subsolid nodules | 1B |
| Solid nodules>8mm | |
| Should be classified according to PM: low (<5%), intermediate or high (>65%) | 2C |
| SPN with low PM: radiological observation | 2C |
| Radiological observation: low-radiation CT without contrast at 3–6, 9–12 and 24 months | 2C |
| SPN with intermediate PM: PET-CT | 1B |
| Negative PET-CT: radiological observation | 2C |
| SPNs with intermediate PM: biopsy-aspiration is an acceptable alternative | 2C |
| No histological diagnosis: PET-CT | 1B |
| Biopsy or aspiration is advisable in case of discordance between clinical PM and imaging tests | 2C |
| Suspected etiology requiring medical treatment (e.g. tuberculosis) | |
| Patients refusing or objecting to diagnostic surgery | |
| SPN with high PM: management according to presumed diagnosis of early stage carcinoma | 2C |
| SPN with high PM: FB with bronchial examination and transbronchial biopsy | 2D |
| Non-diagnosed SPN with PM greater than low in operable patients: SPN resection | 2C |
| Recommended technique: video-assisted thoracoscopy | 1C |
| Interoperative biopsy to establish type of resection | |
| Subcentimeter PNs associated with SPN should not be contraindication for curative carcinoma surgery unless there is confirmation of metastasis. | 2C |
| SPN<8mm | |
| Observation strategy following recommendations of the Fleischner Society (Fig. 1) | 2C |
| Low-dose, non-contrast CT surveillance | 1C |
| Subsolid SPNs | |
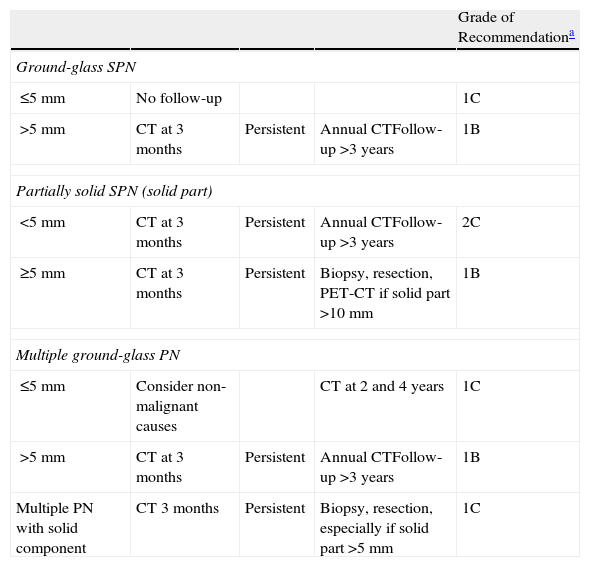
| Intervention according to recommendations of the Fleischner Society (Table 2) | 1B to 2C |
| Low-dose, non-contrast CT surveillance | 1C |
FB: fibroscopy; PN: pulmonary nodule; SPN: solitary pulmonary nodule; PET: positron emission tomography; PM: probability of malignancy; CT: computed tomography.
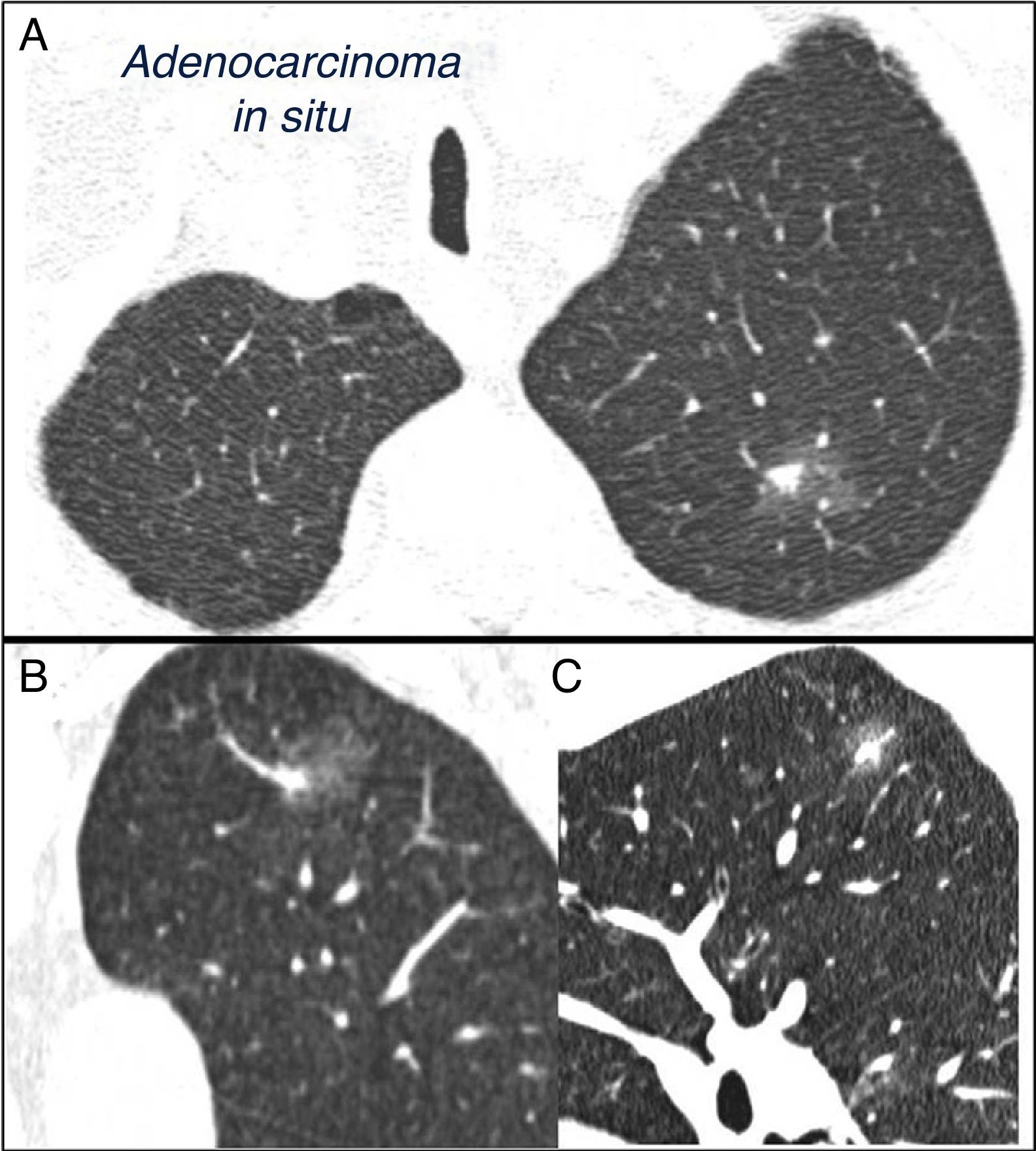
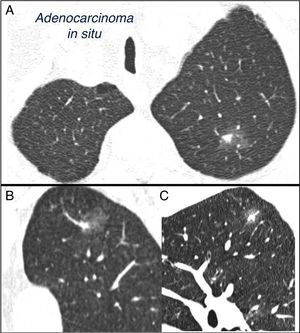
SPN is defined as a single, spherical, distinct, radiological opacity with a long axis of ≤30mm, primarily surrounded by aerated lung and without associated atelectasis, hilar enlargement or pleural effusion.1–3 SPNs may be observed on chest X-ray or chest computed tomography (CT) performed to study other diseases or for diagnostic screening for lung cancer (LC).3,6,7 Millimetric nodules (≤8mm in diameter)1,2,6 and subsolid nodules (SSNs)2,5 (Fig. 4), requiring different management,5–7 may be detected on CT (Fig. 1). SSNs include both ground glass nodules and partially solid nodules that combine a ground glass component with a solid component.2,5–7
Mixed subsolid solitary pulmonary nodule in the left upper lobe. Chest computed tomography with 2mm thin sections in a patient with a solitary pulmonary nodule in the left upper lobe. Axial slice (A) in upper lobes and coronal (B) and sagittal (C) reconstructions in the left upper lobe. An 18-mm ground-glass solitary pulmonary nodule is observed, with a 7-mm solid component in the interior. Resection by video-assisted thoracoscopy revealed adenocarcinoma.
Nodules are estimated to occur at a rate of 1 or 2 per 1000 X-rays.1,3,8 The prevalence of nodules on CTs performed in adult smokers for LC screening is very high, between 20% and 50%, and are generally less than 10mm in size; the incidence in successive annual CTs is 10%.1,3,6,7
When evaluating SPNs with CT, other small nodules are often found. When they are few or one is clearly dominant, they should be considered as independent SPNs, since, even if the situation is LC, accompanying subcentimeter nodules are commonly benign.1,2,6,7 Moreover, in up to 20% of cases, the malignant nodule is not the largest.7
SPN is the radiological manifestation of many diseases3 (online Appendix–Table 1S). However, most nodules are caused by pulmonary malignancies, granulomas and hamartomas.1 The prevalence of malignancy varies between series8,9: from between 5% and 70%, depending on whether the figures are retrieved from health checks or studies of diagnostic techniques, and to a lesser extent in SPNs detected in LC screening programs, where the prevalence is 1%–10%, depending on nodule size.3,7 The PM of SPNs increases notably in patients with previous tumor disease.3,10
Most malignant SPNs are LC, most frequently adenocarcinomas and large cell carcinomas.2,3 In malignant SSN, the most common strains are in the adenocarcinoma spectrum,5,11,12 ranging between atypical adenomatous hyperplasia in the smallest nodules, adenocarcinoma in situ, minimally invasive adenocarcinoma and invasive adenocarcinomas, if a solid component is present, particularly in mucinous nodules and those with lepidic growth.5,11,12
Diagnostic Techniques in the Study of Solitary Pulmonary NoduleChest Computed TomographyThis technique is vastly superior to standard X-ray in the evaluation of SPNs.2,3 It can detect other nodules and mediastinal lymphadenopathies, diagnose pseudonodules (extraparenchymal lesions) and help in planning nodule biopsy or aspiration. In some cases, it can provide a specific diagnosis, such as arteriovenous malformations, mycetomas, rounded atelectasis or hamartomas. Accordingly, CT is essential as the index examination for the study of SPNs.2
Nodule enhancement or contrast material uptake showed a sensitivity of 98% for malignancy with a cutoff of >15 Hounsfield units (HU) and a specificity of 58%.13 This is applicable to spherical, homogeneous SPNs>8mm, without fat, calcium cavitation or necrosis. This may be valuable in centers with expertise in this technique, but due to the introduction of PET its use has not become widespread.3
Positron Emission TomographyOne of the major indications for PET-CT with 18F-deoxy-d-glucose is the study of SPNs. Mean sensitivity for solid SPNs>10–15mm is 0.93 (confidence interval [CI] 0.90–0.95), and mean specificity is 0.8 (CI 0.74–0.85).2,14,15 False negatives on PET-CT are associated with defective technique, tumor diameter<7mm, carcinoid tumors, subsolid nodules and some adenocarcinomas, particularly in situ, minimally invasive, lepidic growth, or mucinous adenocarcinomas.2,3 False positives are more common and include inflammatory and infectious lesions, such as granulomas, tuberculosis, mycosis or pneumonias.2,16
PET-CT is of most use in SPNs>8mm with intermediary PM: a negative study greatly reduces PM.3 In a positive PET-CT, a greater standardized uptake value (SUV) indicates greater tumor aggressivity and a poorer prognosis for the patient, although its reduced specificity, and thus the chance of a false positive, must be taken into account.17,18 It can also help select the most efficient and accessible site for biopsy and help, if necessary, in planning radiation therapy.19 PET-CT contributes to cancer staging2,3 by evaluating mediastinal and systemic metastasis, and is recommended in many LC management guidelines.17,18,20 In these recommendations, PET-CT is used in two ways: as a tool for characterizing the SPN and as a staging technique in SPNs with high PM.
Cytohistological Sampling of Solitary Pulmonary NoduleComputed Tomography, Radioscopy or Ultrasound-Guided Fine Needle Transthoracic AspirationAn analysis of the literature on 48 studies shows good sensitivity, 86% (CI 84%–88%), for the diagnosis of malignancy and very good specificity, 99% (CI 98%–99%).21 In SPNs smaller than 15mm, sensitivity is lower, at 70%–82%.2,22 In benign disease, specificity is also lower.21 The mean rate of pneumothorax was 15%, of which 7% required drainage.2,21,23 Transthoracic aspiration is contraindicated in cases of poor patient collaboration, very compromised respiratory function or single lung or hemorrhagic diathesis, and in the presence of emphysema or extensive bullae in the region of the nodule. Between 4% and 50% of the results do not provide a diagnosis, and up to 20% are false negatives.2 If PM is high, the rate of true negatives is the same as that of false negatives, so it is not useful for excluding malignancy.2
Fiberoptic Bronchoscopy and Associated TechniquesIn LC, the diagnostic yield of CT-guided transbronchial aspiration (TBA) to target the nodule ranges, according to the series, from 20% to 80%, and is lower in SPNs smaller than 20mm, where the mean yield is 30%.2 In benign SPNs, the yield is 10%. This technique is more effective in larger central nodules (>20mm) using an air bronchogram.2,24 There is little risk of TBA: pneumothorax, 2%,2 and more rarely, hemoptysis or hematomas. Although the diagnostic yield for SPNs is less than that obtained with transthoracic fine needle aspiration-biopsy (FNAB), an endobronchial examination can be carried out before planning LC surgery.25 Ultra-fine bronchoscopies for better access to lesions, guide sheaths for positioning the forceps, radial probe ultrasound endobronchoscopy, electromagnetic navigation bronchoscopy and navigation bronchoscopy are all under evaluation, being techniques that allow the forceps to be guided toward the nodule, thus improving yield.2,26 These techniques have been compared in a meta-analysis26: the combined yield was 70%, better than in previous radioscopy-guided series, and the yields of the individual techniques ranged from 68.5% to 73%, although wide variability and heterogeneity were observed among the studies. The yield was lower in SPNs<20mm, 61% versus 80% in >20mm. Combining techniques may improve the yield somewhat.2 As can be seen from these data, no technique in particular outperforms others in terms of yield, and the recommendation is that each center should use the techniques for which both equipment and expertise are available.
Video-assisted Thoracoscopy and ThoracotomySPNs can be resected using these techniques.2 If the SPNs are small or located deep in the parenchyma, they can be previously dyed or marked with a hookwire, generally with CT-guided transthoracic puncture. The risks of video-assisted thoracoscopy are low and mortality is very rare (less than 1%), morbidity is low, and the diagnostic yield is good, similar to thoracotomy but with lower mortality.2 When the SPN is LC, if the clinical situation of the patient permits, anatomical resection is indicated: in general, lobectomy and mediastinal node dissection are recommended.2,20,27
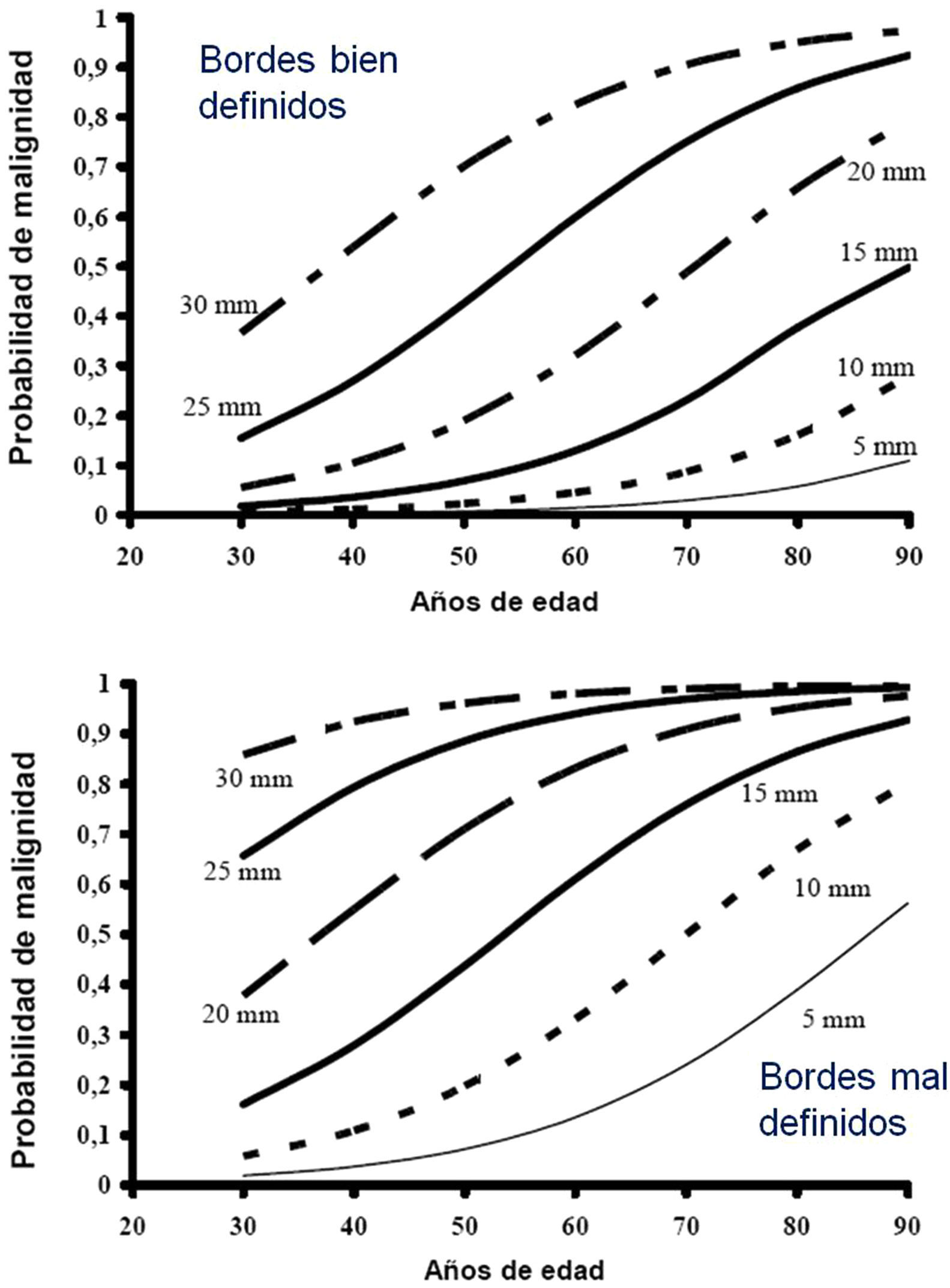
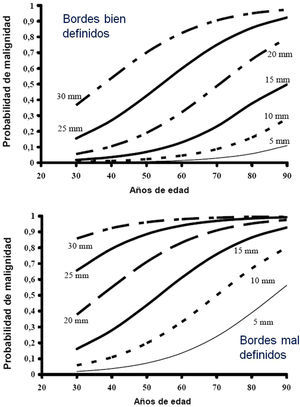
Estimation of the Probability of MalignancyMalignant SPNs differ from benign SPNs in certain clinical and radiological aspects, described in more detail in the extended version of these guidelines (see online supplement). The factors most closely associated with malignancy are the size of the SPN, margin features, density, patient age, accumulated tobacco consumption, existence of other malignancy and detection of growth.2,3,6,7,10,28-30 Central, laminar or total calcification of the SPN is considered a good criterion for benignancy,2,3 as is radiological stability for more than 24 months, implying a doubling time of over 730 days. This criterion is not valid for SSN, for which the observation must be extended to at least 3 years.1–3,5
Most clinicians estimate PM intuitively, but several SPN models and classification rules have been proposed7,28–32 and there are even calculators available online (online Appendix–Table 2S) or as a smartphone application (Medcalc medical calculator). Table 2S of the online Appendix gives a set of formulas obtained from different logistical models and Figure 1S is a valid nomogram for SPNs in chest X-ray.
This estimation of PM guides the subsequent diagnostic process.1,2,31 Logically, when predicting malignancy, the results of all the examination techniques are reviewed, including the PET-CT results,31,32 the biopsy or aspiration results and the evaluation of growth, if suitable images are available.
Along with PM, the foreseeable benefits and risks of the treatment must be assessed. There is a threshold PM indicating observation, i.e., any PM for which the most reasonable option is prospective observation of the stability or growth of the SPN; and a threshold for surgery, for which surgery is clearly recommended since the probability of early stage cancer is high. These thresholds depend on the expected benefits; for example, probability of cure or treatment response in cases of malignant disease, the surgical risks, that may vary between patients and the personal attitude and preference of the patient.1
Sequential Evaluation of the Solitary Pulmonary NoduleThe aim of evaluating an SPN is to diagnose and treat SPNs that represent active disease, in particular LC, since 5-year survival is 70%–80% in early stage disease.20 Another aim is to avoid submitting patients with granulomas, hamartomas and other benign lesions to costly and risky treatments. There is no singly accepted procedure. The key elements for evaluation are estimation of the probability of malignancy, accessibility to different diagnostic tests and the expertise of clinicians in these techniques, and the preferences of the patient.1,2
Figs. 1–3 show the proposed sequential evaluation of SPN: Fig. 1 describes the initial classification by SPN size, density and solidity or subsolidity, separating patients who require individualized management and those who can be diagnosed from the outset. Figs. 2 and 3 describe the proposed strategy for indeterminate solid SPNs>8mm, depending on whether access to PET-CT is easy (Fig. 2) or more difficult (Fig. 3).
Clinical and Initial Radiological Evaluation (1)The initial examination comprises history, examination and the evaluation of the radiological features, along with comparison with all available prior X-rays2,31: grade of recommendation, 1C.
If there is no evidence of stability for >2 years or benign calcification, CT with thin sections through the nodule of interest is indicated2: grade of recommendation, 1B.
Some nodules can be classified in this phase as benign if they are seen to be stable for more than 2 years or from their features on CT2,31: grade of recommendation, 2C.
In indeterminate SPNs, surveillance will continue, depending on the patient characteristics and the radiological features of the nodule.
Patients in Special Situations (2)A patient's situation may be considered special because of a change in the etiological frequency or the PM of the SPN, or because the procedure is limited to non-invasive techniques due to surgical risks.
Patients with prior or concomitant malignant disease. The PM of pulmonary nodules, whether single or multiple, increases greatly in these patients,3,10 even if they are small.6 The possibility of metastasis is even greater if the SPNs were not previously present. Nevertheless, when the SPNs are subcentimetric, up to one-third are benign, so the diagnosis must be confirmed with biopsy, aspiration or video-assisted thoracoscopy before denying potentially curative treatments: grade of recommendation, 2C.2,10 SPNs in immunocompromised patients will require individualized evaluation, and biopsy or aspiration is preferred.33
In inoperable patients, the evaluation of SPN is limited. Biopsy or aspiration is indicated in cases with high PM or positive results on PET, for guiding chemotherapy or radiation therapy if treatment of LC is required: grade of recommendation, 2C.
Solitary Pulmonary Nodule>8mm (3) (Figs. 2 and 3)The first step is to classify the patient according to the estimated PM: low (¿5%), intermediate or high (>65%)1,2,29,31: grade of recommendation, 1C.
Low Probability of Malignancy (3, 1)Low PM is that estimated at less than 5%.2,31 This includes SPNs that are still small (<10–15mm), with distinct margins, younger patients (<40 years), low total tobacco consumption and, of course, no extrapulmonary malignant disease.2,28–32,34 In these cases, radiological observation is recommended2,31: grade of recommendation, 2C.
Also included are SPNs with intermediate PM and negative PET-CT or those evaluated with FNAB showing non-malignancy2: grade of recommendation, 2C.
Intermediate Probability of Malignancy (3, 2)A PM of between 5% and 65% is considered intermediate.2,28–32,34 In these cases, PET-CT, being non-invasive, low risk and of high discriminatory power, is recommended (Fig. 2): grade of recommendation, 1B. A negative result reduces the PM considerably, and observation may be recommended; a positive result increases PM and classifies it as high.2,31
Depending on accessibility and wait times for performing PET-CT, an alternative procedure is CT-guided FNAB or fiberoptic bronchoscopy (FB)-TBA guided by radioscopy, ultrasound endoscopy or electromagnetic or virtual navigation (Fig. 3): grade of recommendation, 2C. The biopsy option is particularly advisable when the clinical PM and findings on imaging tests are discordant, when etiologies requiring specific medical treatment (e.g., tuberculosis) are suspected or if the patient is adverse to surgery2: grade of recommendation, 2C.
Repeating biopsy or aspiration techniques in case of initial negativity is only recommended when the PM of the SPN is high and diagnosis prior to surgery is considered necessary, or when surgery is contraindicated.
If the result is negative, PET-CT would be recommended (Fig. 3): grade of recommendation, 1B. If no PET-CT is available, the alternative would be monitoring with CT, particularly if the FNAB was negative, or surgery.
High Probability of Malignancy (3, 3)PM is high in patients over 50 years of age, with a history of smoking or radiological features of malignancy: SPN>15mm, speculated margins or heterogeneous density. SPNs that are hypermetabolic on PET-CT, those that have increased in size or changed shape and those with a cytology or lung biopsy suggestive of malignancy are also of high PM.2,28–32,34
In these cases, some authors recommend direct diagnostic-therapeutic surgery and others prefer biopsy techniques.2,31 In the population selected by this algorithm, when patients with other cancers have been excluded, most SPNs are lung cancers. Thus, the recommendation is that they are managed as such,2 with PET-CT being recommended as a method for staging17,18: grade of recommendation, 2C.
Pre-surgery histological diagnosis can be determined using FB-TBA, a technique that also allows an evaluation of the bronchial tree before undertaking surgery, or by FNAB. A negative result does not sufficiently reduce the PM to preclude resection of the SPN. The efficacy of attempting pre-surgical diagnosis of SPN with high PM, at clinical stage I or II with CT and PET-CT, provided the patient is operable, has not been investigated. For this reason, this is offered as an option in the algorithm (Fig. 2), although pre-surgical evaluation with FB is a standard practice: grade of recommendation, 2C.
If PET-CT reveals mediastinal or extrathoracic uptakes suggestive of metastasis, these should be evaluated before resection surgery.17,18 If the SPN with high PM is negative on PET-CT, the PM is not sufficiently reduced to recommend observation,2 and resection via video-assisted thoracoscopy is recommended: grade of recommendation, 2C. Lower uptake, however, suggests a better prognosis and less probability of dissemination, something that can be taken into account if the patient has a strong objection to surgery.3,31
In the proposed strategy, the evaluation of an SPN with high PM should conclude with a specific diagnosis or diagnostic-therapeutic resection (Figs. 2 and 3).
Solitary Pulmonary Nodule <8mm (4)The prevalence of these SPNs is very high in CT studies, and PM is low, unless there is a history of previous or concomitant metastasizing tumor.2,6 They are difficult to access for obtaining biopsies and PET-CT and dynamic CT have very low sensitivity.2,6 An observation strategy following the recommendations of the Fleischner Society6 (Fig. 1) is proposed, taking into consideration asymptomatic patients without concomitant malignant disease: grade of recommendation, 2C.
Follow-up is performed with low-dose, non-contrast CT6: grade of recommendation, 1C. Again, the patient must be informed and his/her preferences must be taken into consideration.2
Subsolid Pulmonary Nodule (5)These are ground-glass nodules, pure or with a solid component5 (Fig. 4). Determination of a subsolid nodule requires thin sections, preferably of 1mm, since small solid SPNs in 5-mm CT slices may appear as ground glass.2,5
SSNs are difficult to access for biopsy or aspiration and the sensitivity of PET-CT is low.5,35 On the other hand, their PM is relatively high, 15% or more in the case of ground-glass SPN and over 50% for a mixed nodule.5 It is also more difficult to establish changes in size or volume of these lesions during surveillance, and malignancies that appear in this form may be indolent for an extended period, for which reason the period of observation must be longer,5 i.e., at least 3 years.
As many benign etiologies are acute or subacute processes, an initial observation strategy with CT at 3 months is proposed, since some SPNs can disappear. If they persist, the strategy will be determined by the size of the SPN, if they have a solid component and if they are single or multiple (Fig. 1). Table 2 lists in detail the recommendations as proposed by the Fleischner Society.5
Management of Subsolid Nodules.
| Grade of Recommendationa | ||||
| Ground-glass SPN | ||||
| ≤5mm | No follow-up | 1C | ||
| >5mm | CT at 3 months | Persistent | Annual CTFollow-up >3 years | 1B |
| Partially solid SPN (solid part) | ||||
| <5mm | CT at 3 months | Persistent | Annual CTFollow-up >3 years | 2C |
| ≥5mm | CT at 3 months | Persistent | Biopsy, resection, PET-CT if solid part >10mm | 1B |
| Multiple ground-glass PN | ||||
| ≤5mm | Consider non-malignant causes | CT at 2 and 4 years | 1C | |
| >5mm | CT at 3 months | Persistent | Annual CTFollow-up >3 years | 1B |
| Multiple PN with solid component | CT 3 months | Persistent | Biopsy, resection, especially if solid part >5mm | 1C |
PN: pulmonary nodule; SPN: solitary pulmonary nodule; PET: positron emission tomography; CT: computed tomography. The grade of recommendation is that proposed by the Fleischner Society,5 based on the GRADE system. The recommendations of the American College of Chest Physicians2 reduce the strength of the recommendation by one grade. The CT for evaluation must be fine slice and follow-up low-dose CT.
- A.
Diagnosis of benignancy: Patients with a specific diagnosis on CT, such as hamartomas, arteriovenous malformations, cystic lesions, rounded atelectasis, mycetomas, pseudonodules or calcified nodules, or who have criteria for benignancy, e.g., documented stability for at least 2 years, in the case of solid nodules, or at least 3 years for subsolid nodules2,3: grade of recommendation, 2C.
- B.
Radiological observation: Indicated in SPNs with low PM, or with intermediate PM when PET-CT is negative, grade of recommendation, 2C. This may also be indicated in undiagnosed SPNs if the risk of surgery is very high or if the patient refuses surgery: grade of recommendation, 2C.
For SPN>8mm with low PM, CT monitoring is recommended at 3–6 months, again at 9–12 months and again at 24 months.2 There are specific strategies for SPN≤8mm and SSN2,5,6 (Fig. 1 and Table 2): grade of recommendation, 2C. Surveillance CTs must be performed with low-dose and non-contrast: grade of recommendation, 1C.
- C.
Diagnostic-therapeutic surgery: Operable patients with undiagnosed SPN and PM greater than low should be offered resection: grade of recommendation, 2C. Although video-assisted thoracoscopy is the method of choice (grade of recommendation, 1C), each surgical team will decide on the best approach. Intraoperative biopsy is recommended for completing appropriate resection in the case of LC: grade of recommendation, 1C.
- D.
Specific diagnosis: This is the etiological diagnosis of SPN. If LC is diagnosed, specific local staging and treatment protocols will be applied.20
The algorithm cannot be completed without re-emphasizing that the optimal decision must include the opinion and the preferences of the appropriately informed patient2: grade of recommendation, 1C.
The authors state that they have no conflict of interests.
Please cite this article as: Álvarez Martínez CJ, Bastarrika Alemañ G, Disdier Vicente C, Fernández Villar A, Hernández Hernández JR, Maldonado Suárez A, et al. Normativa sobre el manejo del nódulo pulmonar solitario. Arch Bronconeumol. 2014;50:285–293.