Electromagnetic navigation bronchoscopy (ENB) and radial endobronchial ultrasound (R-EBUS) are advanced imaging-guided bronchoscopy techniques for diagnosing pulmonary lesions. This study aimed to determine the comparative diagnostic yield of sole ENB and R-EBUS under moderate sedation.
MethodsWe investigated 288 patients who underwent sole ENB (n=157) or sole R-EBUS (n=131) under moderate sedation for pulmonary lesion biopsy between January 2017 and April 2022. After a 1:1 propensity score-matching to control for pre-procedural factors, the diagnostic yield, sensitivity for malignancy, and procedure-related complications between both techniques were compared.
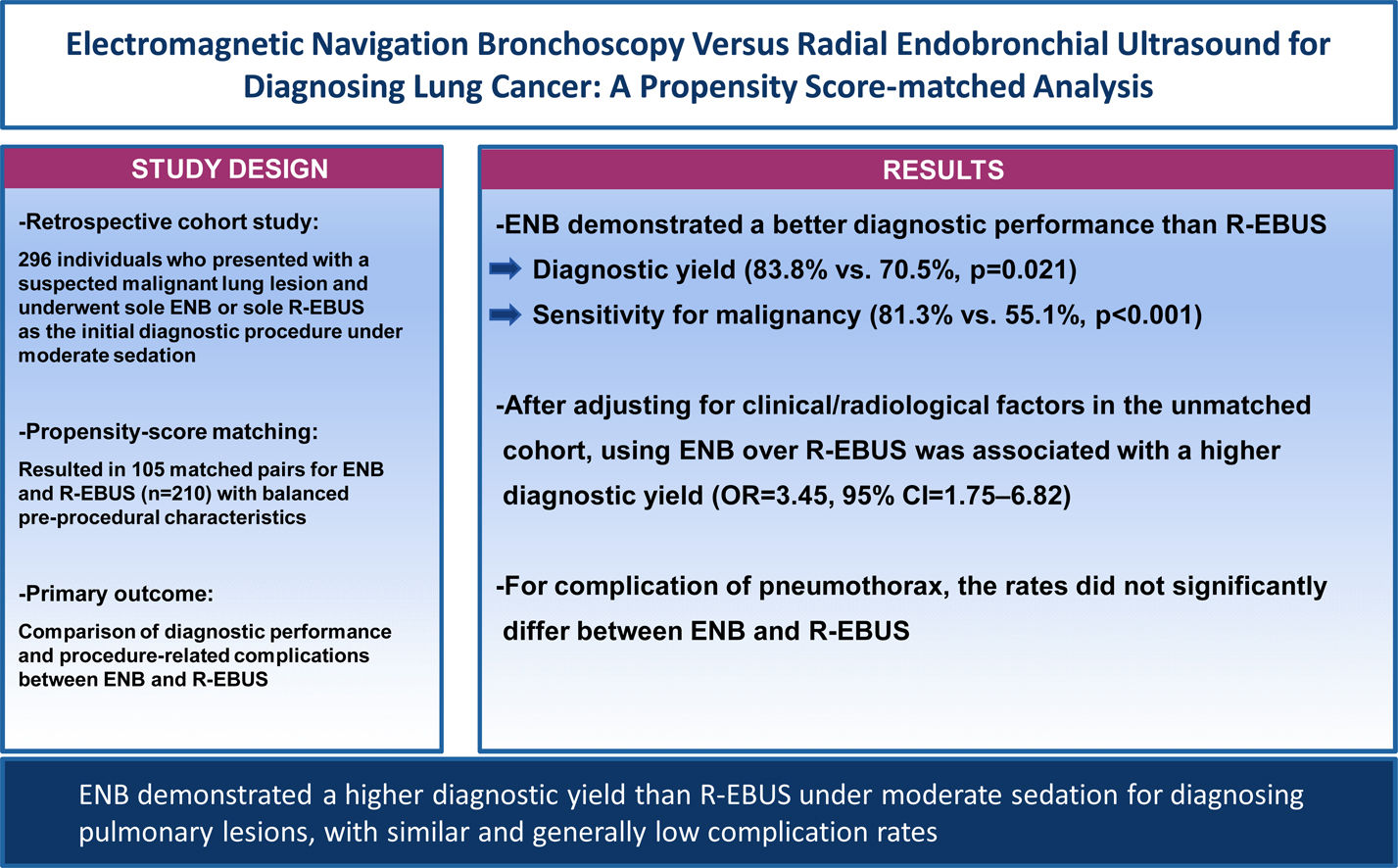
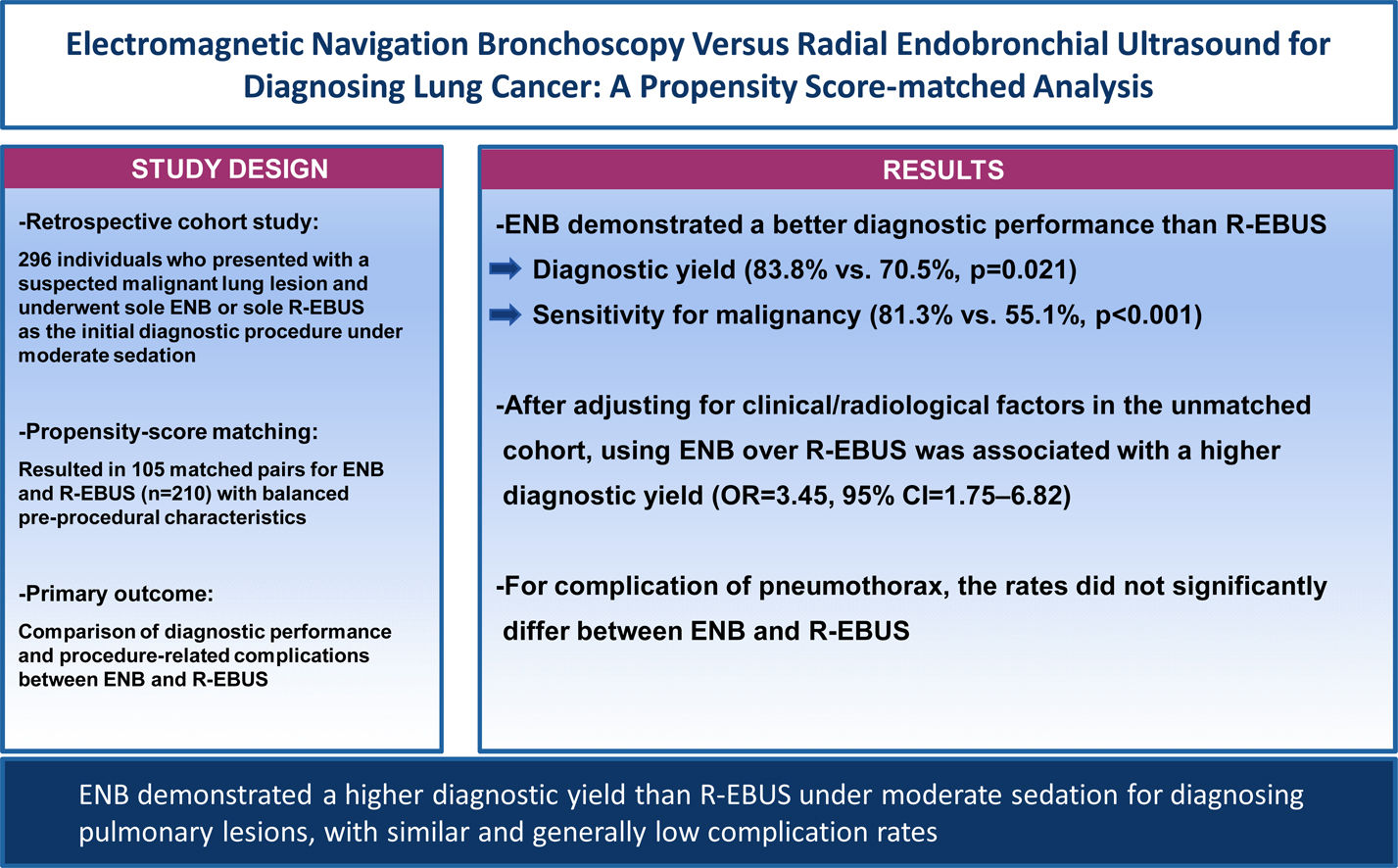
ResultsThe matching resulted in 105 pairs/procedure for analyses with balanced clinical and radiological characteristics. The overall diagnostic yield was significantly higher for ENB than for R-EBUS (83.8% vs. 70.5%, p=0.021). ENB demonstrated a significantly higher diagnostic yield than R-EBUS among those with lesions>20mm in size (85.2% vs. 72.3%, p=0.034), radiologically solid lesions (86.7% vs. 72.7%, p=0.015), and lesions with a class 2 bronchus sign (91.2% vs. 72.3%, p=0.002), respectively. The sensitivity for malignancy was also higher for ENB than for R-EBUS (81.3% vs. 55.1%, p<0.001). After adjusting for clinical/radiological factors in the unmatched cohort, using ENB over R-EBUS was significantly associated with a higher diagnostic yield (odd ratio=3.45, 95% confidence interval=1.75–6.82). Complication rates for pneumothorax did not significantly differ between ENB and R-EBUS.
ConclusionENB demonstrated a higher diagnostic yield than R-EBUS under moderate sedation for diagnosing pulmonary lesions, with similar and generally low complication rates. Our data indicate the superiority of ENB over R-EBUS in a least-invasive setting.
Lung cancer is presently the leading cause of cancer-related mortality worldwide.1 Widespread efforts have been made to effectively detect and diagnose lung cancer.2–4 Recently, the wide implementation of lung cancer screening with low-dose chest computed tomography has considerably increased the identification of suspicious pulmonary lesions that require diagnostic evaluation.5,6 Diagnosing pulmonary lesions involves the use of various techniques, including bronchoscopy, transthoracic approach, and surgery. According to clinical practice guidelines, the least invasive approach with the highest yield is recommended for diagnosing pulmonary lesions suspicious for lung cancer.7–9 For peripheral lesions difficult to reach with conventional bronchoscopy, transthoracic needle aspiration (TTNA) has been commonly adopted. However, despite a good sensitivity of approximately 90%, complications are not uncommon with TTNA, with a reported pneumothorax rate of >18% of the biopsied cases.10,11
Recently, advances in image-assisted technologies using flexible bronchoscopy have improved diagnostic yield and safety. Leading bronchoscopy techniques include electromagnetic navigation bronchoscopy (ENB) and radial endobronchial ultrasound (R-EBUS).12 ENB and R-EBUS allow physicians to access peripheral lung lesions through a minimally invasive technique using a dedicated navigation software system (ENB) or a 20-MHz ultrasound probe (R-EBUS), respectively. According to current guidelines, ENB or R-EBUS is recommended for pulmonary lesions that cannot be reached with conventional flexible bronchoscopy alone.7,13 For ENB, one large multicenter prospective study,14 followed by a recent meta-analysis,15 has demonstrated that ENB-guided diagnosis has a diagnostic yield of 68–77%. Meanwhile, R-EBUS has been reported to have a diagnostic accuracy of approximately 70%.16,17 Both techniques provide an acceptable performance for diagnosing lung cancer, with markedly lower complication rates than TTNA.18
However, to date, information on patient-level data comparing ENB and R-EBUS, especially when performed under moderate sedation, is limited. Despite the theoretical advantages on localization and the higher diagnostic yield of ENB than R-EBUS demonstrated in separate pooled-analyses,15,17–19 a direct comparison of the two procedures are rarely available with uncontrolled lesion characteristics and sedation methods.12,18 Therefore, we aimed to evaluate and compare the diagnostic performance and complication rates of ENB and R-EBUS under moderate sedation for diagnosing pulmonary lesions suspicious for lung cancer. We performed propensity score matching between patients who underwent ENB and those who underwent R-EBUS including lesion characteristics to control for pre-procedural factors and address selection bias.
MethodsStudy design and participantsThis study was conducted in Seoul National University Bundang Hospital, a large, university-affiliated, tertiary-care center where sole ENB and R-EBUS techniques were available from 2017. We identified consecutive individuals presenting with a suspected malignant lung lesion that required pathological evaluation and candidates for an elective ENB or R-EBUS as the initial diagnostic procedure between January 2017 and April 2022. In the institution, the routine procedure for guided bronchoscopic biopsy involved the use of sole ENB or sole R-EBUS under moderate sedation without additional complementary tools, such as fluoroscopy. During the study period, advanced bronchoscopic techniques that are not compatible with moderate sedation (e.g., robot-assisted bronchoscopy) were not accessible. The choice of modality (ENB or R-EBUS) was based on the discretion of the attending specialist in the pulmonary division. Patients who underwent ENB or R-EBUS under general anesthesia owing to unavoidable medical conditions or intraoperative studies were excluded. The study design was approved by the institutional review board of the Seoul National University Bundang Hospital (IRB no:B-2112-729-101), which waived the need for written informed consent from participants.
ENB and R-EBUS proceduresFor those scheduled for ENB, on the day of the procedure, all participants underwent inspiration/expiration chest computed tomography (CT) to index with navigation platforms and reconstruct virtual airway routes prior to the procedure. All ENB procedures were performed using the Spin Thoracic Navigation System (SYS-4230K; Veran Medical, St. Louis, MO, USA). Biopsy tools used by the performing physicians were aspirating needles and biopsy forceps for the ENB-guided biopsy. All R-EBUS procedures were performed using the 20-MHz mechanical radial-type probe (outer diameter, 1.7mm, UM-S20-17S; Olympus Corporation, Tokyo, Japan) with or without a guide sheath (outer diameter, 2mm, K201; Olympus Corporation, Tokyo, Japan). Biopsy forceps were used to obtain tissues in the R-EBUS-guided biopsy. Rapid on-site examination (ROSE) was not performed in any of the cases. Mediastinal staging of lung cancer with linear EBUS could be performed after the guided-biopsy procedure at the attending physician's discretion. When sequential nodal staging was performed with additional linear EBUS, nodal biopsy results were evaluated independently from the results from pulmonary lesions biopsied by ENB or R-EBUS.
All ENB and R-EBUS procedures were performed by one of the nine pulmonologists (YWK, HJK, MJS, BSK, SYL, YJL, JSP, YJC, and JHL each of whom had at least 3 years of experience with advanced bronchoscopic biopsy procedures). Bronchoscopy was performed under moderate sedation by administration of 2–3mg of midazolam and 25–50μg of fentanyl intravenously at the onset of the procedure and local anesthesia with topical 1% lidocaine. The physician conducting the procedure may have administered an additional dose of midazolam or fentanyl during the procedure for adequate sedation. A bronchoscope with an outer diameter of 5.9mm (BF-1TQ290), 4.9mm (BF-260), or 4.0mm (BF-P260F) (all Olympus Corporation, Tokyo, Japan) was used for the ENB or R-EBUS procedures. Other than the sole use of ENB or R-EBUS, no other specified restrictions on additional procedural techniques were implemented (e.g., additional cytology brushing, bronchoalveolar lavage, or bronchial washing), and the procedures were subjected to the performing physician's discretion.
Lesion characteristics and outcome measuresMedical records documenting clinical data, including demographic characteristics, radiologic findings, procedure reports, pathological findings, the final diagnoses of the biopsied lesion, and procedure-associated complications were obtained and analyzed. For radiologic findings, a pulmonary physician (YWK) and a radiologist (SHY) who were blinded to procedural results reviewed all the CT images taken prior to the procedure. The presence of a bronchus sign on CT was determined and classified as follows: class 0: the absence of a bronchus sign, class 1: an airway immediately adjacent to the lesion, and class 2: an airway directly aligned with the lesion.20,21 For images with inconsistent findings, consensus was reached through discussion.
Pathological results of biopsied samples that revealed non-malignant or indeterminate conditions were initially considered negative. For such cases, the attending physician made decisions regarding the follow-up and attempt to perform other invasive procedures for pathological evaluations. When additional biopsy was needed, non-bronchoscopic procedures, such as TTNA or surgical resection, were considered preferentially. The following instances were defined as false negatives: (1) a repeat biopsy (e.g., TTNA, bronchoscopic, and surgical) that proved malignancy, (2) lesion growth observed on follow-up CT imaging within 12 months, (3) treated as lung cancer without pathological confirmation, and (4) lung cancer diagnosed from other sites within 3 months (including diagnosis at metastatic lymph nodes by sequential linear EBUS). Meanwhile, the following cases were defined as true negatives: (1) subsequent diagnostic tests confirmed a non-malignant diagnosis, (2) the lesion resolved without lung cancer treatment, and (3) no progression on CT follow-up up to 12 months. Procedure-related complications were determined and classified according to the Common Terminology Criteria for Adverse Events (CTCAE) scale, ver. 5.0.22 Pneumothorax was confirmed by chest X-rays performed post-procedurally and a day after the procedure. Severe bronchopulmonary hemorrhage was considered when blood transfusion or intervention was required. The primary outcomes were the diagnostic yield of ENB and R-EBUS, and safety endpoints were defined as the incidence and severity of associated complications.
Statistical analysisThe characteristics of the participants are presented as frequencies (%) and as means and standard deviations for categorical and continuous variables, respectively. To account for differences in pre-procedural patient and lesion characteristics between the ENB group and R-EBUS group, a propensity score model was generated using logistic regression.23 Information on propensity matching is available in the Supplementary Methods.
The diagnostic yield was calculated for each group as the sum of the rate of true-positive cases for malignancy and that of true-negative cases of all participants who underwent attempted guided-biopsy. Only the biopsy results of the pulmonary lesion by ENB or R-EBUS were evaluated for the diagnostic yield and molecular testing. We assumed indeterminate cases that had initially negative results with insufficient follow-up duration of 12 months to conclude the final diagnosis as false negatives, which provided the low estimates of the diagnostic yield, sensitivity, specificity, positive and negative predictive values of both techniques for diagnosing malignancy. Comparisons of these values, complication rates, and categorical variables were performed with a chi-square test or a Fisher's exacts test as appropriate. Continuous variables were compared using an independent t-test.24 Logistic regression analysis was conducted to determine the association between modality choice and the diagnostic yield in the unmatched and matched cohorts. For the unmatched cohort, multivariable models were constructed with the inclusion of age, sex, smoking pack-years, and other variables (lesion size, solidity, upper/lower lobe distribution, distance from the pleura, bronchus sign class, presence of emphysema or interstitial fibrosis, subsequent linear EBUS, and total procedure time) with statistically significant associations in univariable analyses. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs), and p-values<0.05 were considered statistically significant. All analyses were performed using R version 3.5.3 (http://www.R-projecct.org) and STATA, version 16.0 (StataCorp., College Station, TX, USA).
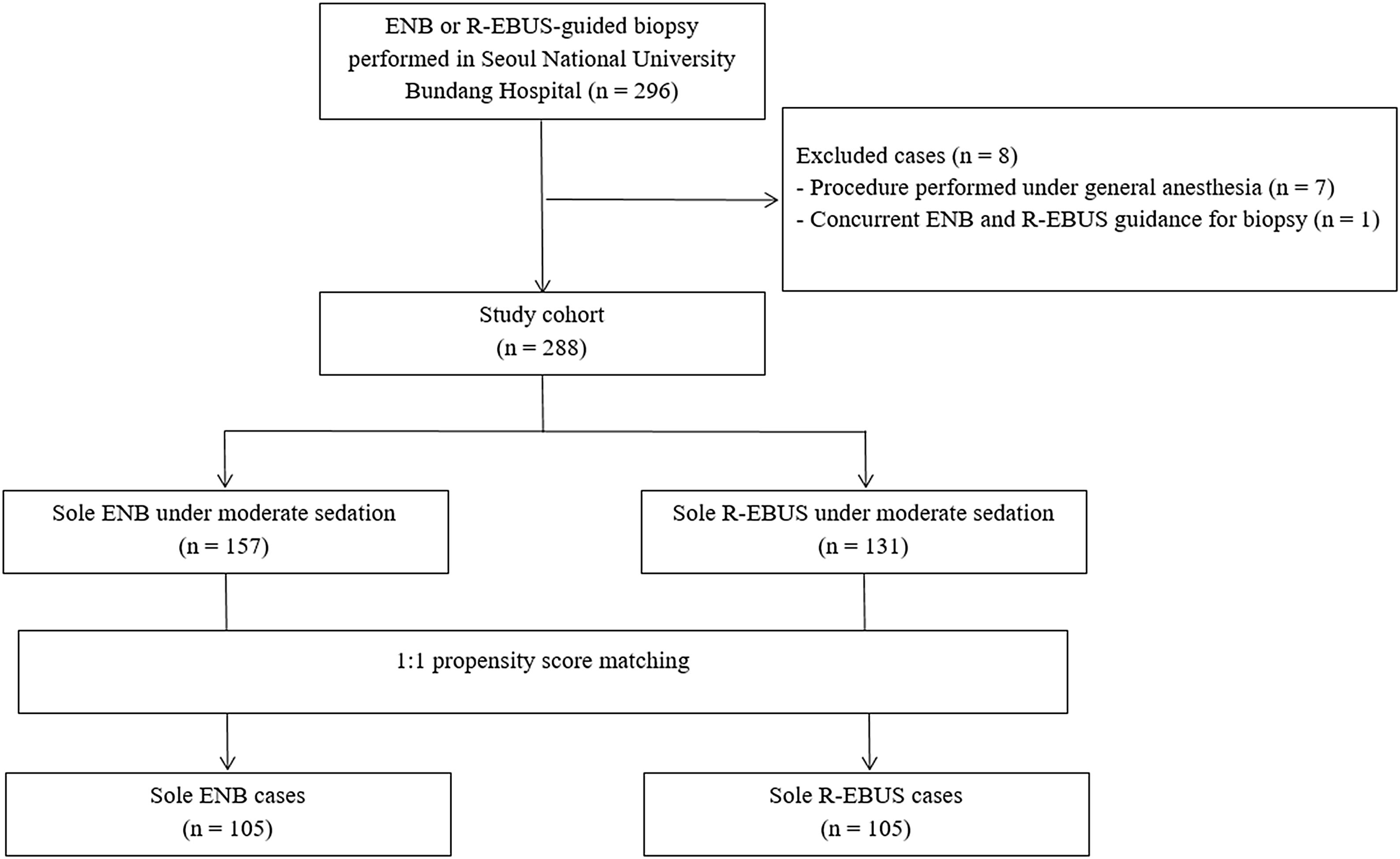
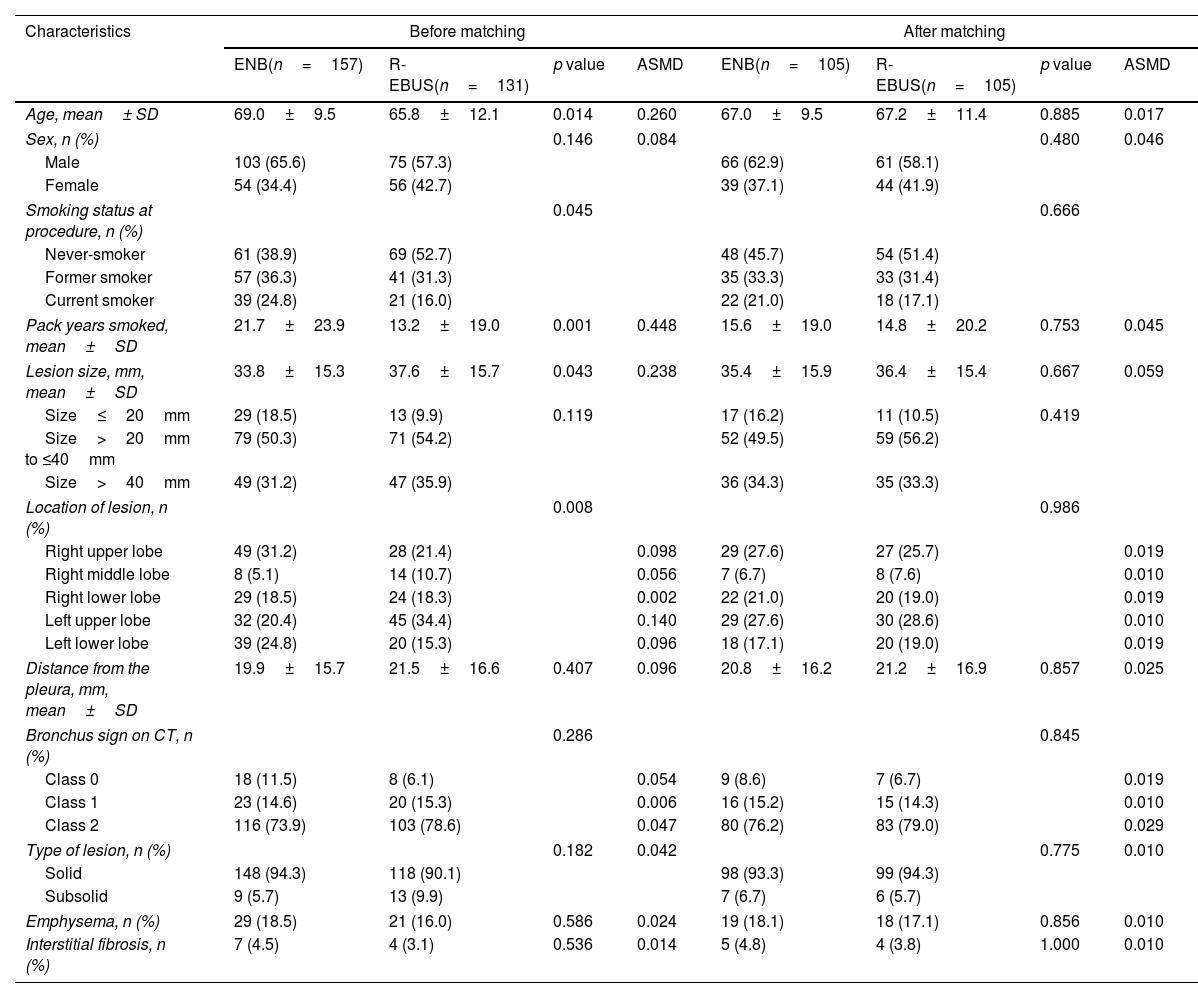
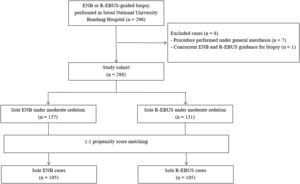
ResultsPatient characteristics before and after propensity score matchingFig. 1 depicts the study flowchart. During the study period, 296 individuals underwent ENB or R-EBUS. After applying the exclusion criteria, 288 participants (157 who underwent sole ENB and 131 who underwent sole R-EBUS under moderate sedation) were included. Table 1 lists the pre-procedural clinical and radiologic characteristics and the differences between the sole ENB group and sole R-EBUS group before and after propensity score matching. The 1:1 matching resulted in 105 matched pairs (n=210) with balanced characteristics (ASMD<0.1, Supplementary Fig. 1) intended for adjustment.
Pre-procedural demographic and radiologic characteristics and difference between electromagnetic navigation and radial ultrasound bronchoscopy before and after propensity score matching.
| Characteristics | Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|---|
| ENB(n=157) | R-EBUS(n=131) | p value | ASMD | ENB(n=105) | R-EBUS(n=105) | p value | ASMD | |
| Age, mean± SD | 69.0±9.5 | 65.8±12.1 | 0.014 | 0.260 | 67.0±9.5 | 67.2±11.4 | 0.885 | 0.017 |
| Sex, n (%) | 0.146 | 0.084 | 0.480 | 0.046 | ||||
| Male | 103 (65.6) | 75 (57.3) | 66 (62.9) | 61 (58.1) | ||||
| Female | 54 (34.4) | 56 (42.7) | 39 (37.1) | 44 (41.9) | ||||
| Smoking status at procedure, n (%) | 0.045 | 0.666 | ||||||
| Never-smoker | 61 (38.9) | 69 (52.7) | 48 (45.7) | 54 (51.4) | ||||
| Former smoker | 57 (36.3) | 41 (31.3) | 35 (33.3) | 33 (31.4) | ||||
| Current smoker | 39 (24.8) | 21 (16.0) | 22 (21.0) | 18 (17.1) | ||||
| Pack years smoked, mean±SD | 21.7±23.9 | 13.2±19.0 | 0.001 | 0.448 | 15.6±19.0 | 14.8±20.2 | 0.753 | 0.045 |
| Lesion size, mm, mean±SD | 33.8±15.3 | 37.6±15.7 | 0.043 | 0.238 | 35.4±15.9 | 36.4±15.4 | 0.667 | 0.059 |
| Size≤20mm | 29 (18.5) | 13 (9.9) | 0.119 | 17 (16.2) | 11 (10.5) | 0.419 | ||
| Size>20mm to ≤40mm | 79 (50.3) | 71 (54.2) | 52 (49.5) | 59 (56.2) | ||||
| Size>40mm | 49 (31.2) | 47 (35.9) | 36 (34.3) | 35 (33.3) | ||||
| Location of lesion, n (%) | 0.008 | 0.986 | ||||||
| Right upper lobe | 49 (31.2) | 28 (21.4) | 0.098 | 29 (27.6) | 27 (25.7) | 0.019 | ||
| Right middle lobe | 8 (5.1) | 14 (10.7) | 0.056 | 7 (6.7) | 8 (7.6) | 0.010 | ||
| Right lower lobe | 29 (18.5) | 24 (18.3) | 0.002 | 22 (21.0) | 20 (19.0) | 0.019 | ||
| Left upper lobe | 32 (20.4) | 45 (34.4) | 0.140 | 29 (27.6) | 30 (28.6) | 0.010 | ||
| Left lower lobe | 39 (24.8) | 20 (15.3) | 0.096 | 18 (17.1) | 20 (19.0) | 0.019 | ||
| Distance from the pleura, mm, mean±SD | 19.9±15.7 | 21.5±16.6 | 0.407 | 0.096 | 20.8±16.2 | 21.2±16.9 | 0.857 | 0.025 |
| Bronchus sign on CT, n (%) | 0.286 | 0.845 | ||||||
| Class 0 | 18 (11.5) | 8 (6.1) | 0.054 | 9 (8.6) | 7 (6.7) | 0.019 | ||
| Class 1 | 23 (14.6) | 20 (15.3) | 0.006 | 16 (15.2) | 15 (14.3) | 0.010 | ||
| Class 2 | 116 (73.9) | 103 (78.6) | 0.047 | 80 (76.2) | 83 (79.0) | 0.029 | ||
| Type of lesion, n (%) | 0.182 | 0.042 | 0.775 | 0.010 | ||||
| Solid | 148 (94.3) | 118 (90.1) | 98 (93.3) | 99 (94.3) | ||||
| Subsolid | 9 (5.7) | 13 (9.9) | 7 (6.7) | 6 (5.7) | ||||
| Emphysema, n (%) | 29 (18.5) | 21 (16.0) | 0.586 | 0.024 | 19 (18.1) | 18 (17.1) | 0.856 | 0.010 |
| Interstitial fibrosis, n (%) | 7 (4.5) | 4 (3.1) | 0.536 | 0.014 | 5 (4.8) | 4 (3.8) | 1.000 | 0.010 |
ASMD, absolute standardized mean difference; ENB, electromagnetic navigation bronchoscopy; CT, computed tomography; R-EBUS, radial endobronchial ultrasound; SD, standard deviation.
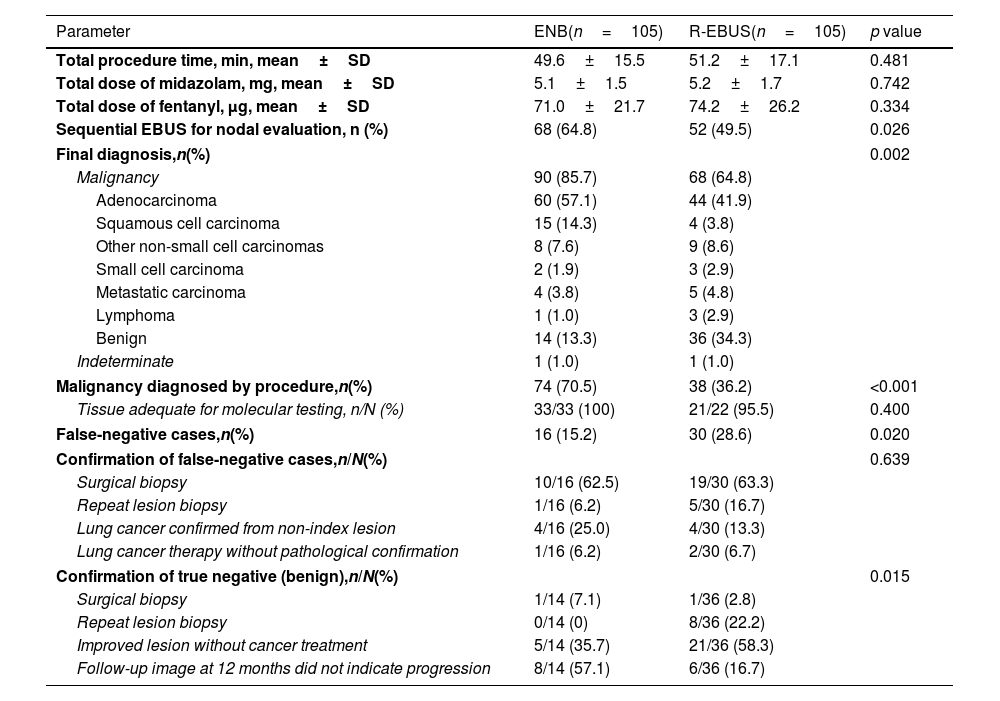
Table 2 describes the procedural characteristics and diagnostic results of those who underwent ENB or R-EBUS in the matched cohort. No significant differences in the total procedure time, total dose of midazolam and fentanyl used to achieve moderate sedation were observed between the patients who underwent ENB and R-EBUS. Of those who underwent guided biopsy, 90 (85.7%) and 68 (64.8%) were finally diagnosed with malignancy in the ENB group and R-EBUS group, respectively (p=0.002). For both groups, lung adenocarcinoma was the major histologic type of cancer. The proportion of malignancy diagnosed by procedure was 70.5% for ENB and 36.2% for R-EBUS (p<0.001). The lesions in 16 patients (15.2%) in the ENB group and 30 patients (28.6%) in the R-EBUS group were initially considered non-malignant or indeterminate by procedure but were eventually diagnosed as malignant (false negative, p=0.020). In both groups, surgical biopsy was the most common method used to confirm malignancy in initially false negatives.
Matched comparison of procedural characteristics, diagnostic results, methods of confirmation between electromagnetic navigation and radial ultrasound bronchoscopy.
| Parameter | ENB(n=105) | R-EBUS(n=105) | p value |
|---|---|---|---|
| Total procedure time, min, mean±SD | 49.6±15.5 | 51.2±17.1 | 0.481 |
| Total dose of midazolam, mg, mean±SD | 5.1±1.5 | 5.2±1.7 | 0.742 |
| Total dose of fentanyl, μg, mean±SD | 71.0±21.7 | 74.2±26.2 | 0.334 |
| Sequential EBUS for nodal evaluation, n (%) | 68 (64.8) | 52 (49.5) | 0.026 |
| Final diagnosis,n(%) | 0.002 | ||
| Malignancy | 90 (85.7) | 68 (64.8) | |
| Adenocarcinoma | 60 (57.1) | 44 (41.9) | |
| Squamous cell carcinoma | 15 (14.3) | 4 (3.8) | |
| Other non-small cell carcinomas | 8 (7.6) | 9 (8.6) | |
| Small cell carcinoma | 2 (1.9) | 3 (2.9) | |
| Metastatic carcinoma | 4 (3.8) | 5 (4.8) | |
| Lymphoma | 1 (1.0) | 3 (2.9) | |
| Benign | 14 (13.3) | 36 (34.3) | |
| Indeterminate | 1 (1.0) | 1 (1.0) | |
| Malignancy diagnosed by procedure,n(%) | 74 (70.5) | 38 (36.2) | <0.001 |
| Tissue adequate for molecular testing, n/N (%) | 33/33 (100) | 21/22 (95.5) | 0.400 |
| False-negative cases,n(%) | 16 (15.2) | 30 (28.6) | 0.020 |
| Confirmation of false-negative cases,n/N(%) | 0.639 | ||
| Surgical biopsy | 10/16 (62.5) | 19/30 (63.3) | |
| Repeat lesion biopsy | 1/16 (6.2) | 5/30 (16.7) | |
| Lung cancer confirmed from non-index lesion | 4/16 (25.0) | 4/30 (13.3) | |
| Lung cancer therapy without pathological confirmation | 1/16 (6.2) | 2/30 (6.7) | |
| Confirmation of true negative (benign),n/N(%) | 0.015 | ||
| Surgical biopsy | 1/14 (7.1) | 1/36 (2.8) | |
| Repeat lesion biopsy | 0/14 (0) | 8/36 (22.2) | |
| Improved lesion without cancer treatment | 5/14 (35.7) | 21/36 (58.3) | |
| Follow-up image at 12 months did not indicate progression | 8/14 (57.1) | 6/36 (16.7) | |
ENB, electromagnetic navigation bronchoscopy; R-EBUS, radial endobronchial ultrasound; SD, standard deviation; EBUS, endobronchial ultrasound.
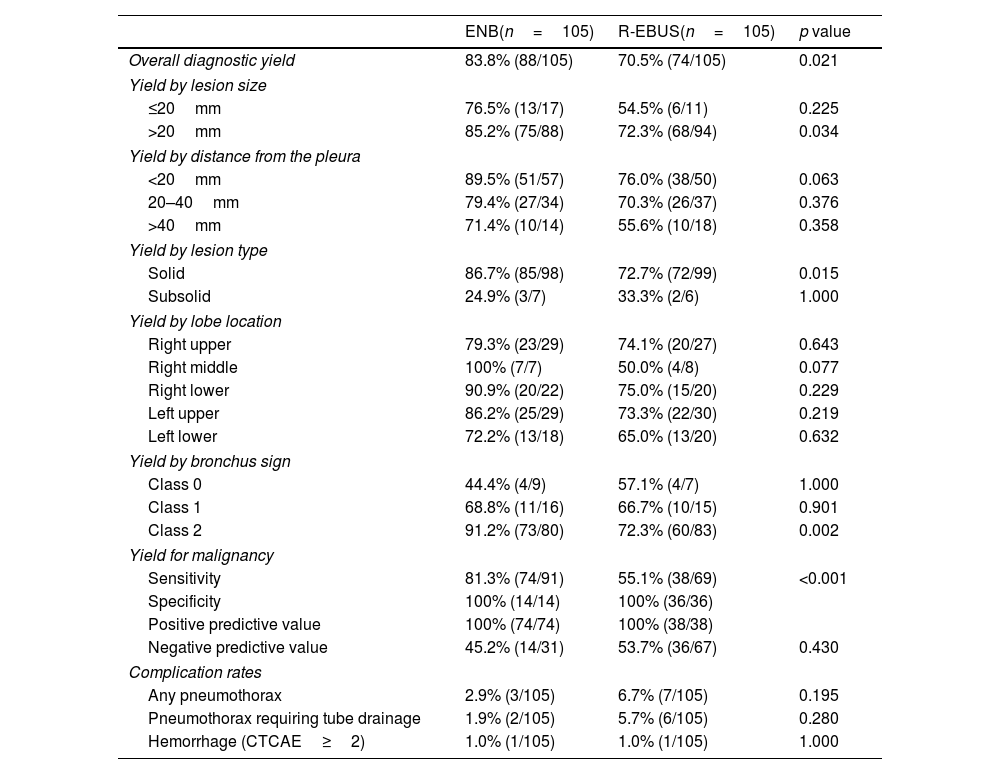
Table 3 describes the matched comparison of the diagnostic yield and complication rates of ENB and R-EBUS. The comparison of the final diagnostic yield was further stratified by lesion characteristics. The overall diagnostic yield was significantly higher in the ENB group than in the R-EBUS group (83.8% vs. 70.5%, p=0.021). By subgroups analyses, ENB demonstrated a significantly higher diagnostic yield than R-EBUS among those with lesions>20mm in size (85.2% vs. 72.3%, p=0.034), radiologically solid lesions (86.7% vs. 72.7%, p=0.015), lesions with a class 2 bronchus sign (91.2% vs. 72.3%, p=0.002), and lesions that were finally diagnosed as malignant (i.e. sensitivity for cancer diagnosis, 81.3% vs. 55.1%, p<0.001). For adverse events, the complication rates for any pneumothorax, pneumothorax requiring tube drainage, bronchopulmonary hemorrhage of CTCAE≥grade 2 did not significantly differ between both groups. When evaluating the initial unmatched cohort, the overall diagnostic yield was 81.5% (128/157) for ENB and 67.9% (89/131) for R-EBUS (p=0.008). The comparison of high estimates of the diagnostic yield, which were calculated by assuming all indeterminate cases as true negatives, are presented in Supplementary Table 1.
Matched comparison of the diagnostic yield and complication rates of ENB and R-EBUS.
| ENB(n=105) | R-EBUS(n=105) | p value | |
|---|---|---|---|
| Overall diagnostic yield | 83.8% (88/105) | 70.5% (74/105) | 0.021 |
| Yield by lesion size | |||
| ≤20mm | 76.5% (13/17) | 54.5% (6/11) | 0.225 |
| >20mm | 85.2% (75/88) | 72.3% (68/94) | 0.034 |
| Yield by distance from the pleura | |||
| <20mm | 89.5% (51/57) | 76.0% (38/50) | 0.063 |
| 20–40mm | 79.4% (27/34) | 70.3% (26/37) | 0.376 |
| >40mm | 71.4% (10/14) | 55.6% (10/18) | 0.358 |
| Yield by lesion type | |||
| Solid | 86.7% (85/98) | 72.7% (72/99) | 0.015 |
| Subsolid | 24.9% (3/7) | 33.3% (2/6) | 1.000 |
| Yield by lobe location | |||
| Right upper | 79.3% (23/29) | 74.1% (20/27) | 0.643 |
| Right middle | 100% (7/7) | 50.0% (4/8) | 0.077 |
| Right lower | 90.9% (20/22) | 75.0% (15/20) | 0.229 |
| Left upper | 86.2% (25/29) | 73.3% (22/30) | 0.219 |
| Left lower | 72.2% (13/18) | 65.0% (13/20) | 0.632 |
| Yield by bronchus sign | |||
| Class 0 | 44.4% (4/9) | 57.1% (4/7) | 1.000 |
| Class 1 | 68.8% (11/16) | 66.7% (10/15) | 0.901 |
| Class 2 | 91.2% (73/80) | 72.3% (60/83) | 0.002 |
| Yield for malignancy | |||
| Sensitivity | 81.3% (74/91) | 55.1% (38/69) | <0.001 |
| Specificity | 100% (14/14) | 100% (36/36) | |
| Positive predictive value | 100% (74/74) | 100% (38/38) | |
| Negative predictive value | 45.2% (14/31) | 53.7% (36/67) | 0.430 |
| Complication rates | |||
| Any pneumothorax | 2.9% (3/105) | 6.7% (7/105) | 0.195 |
| Pneumothorax requiring tube drainage | 1.9% (2/105) | 5.7% (6/105) | 0.280 |
| Hemorrhage (CTCAE≥2) | 1.0% (1/105) | 1.0% (1/105) | 1.000 |
ENB, electromagnetic navigation bronchoscopy; R-EBUS, radial endobronchial ultrasound; CTCAE, Common Terminology Criteria for Adverse Events.
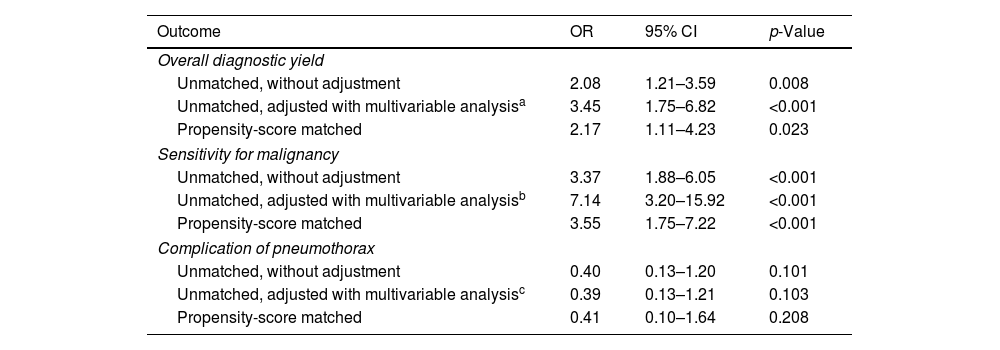
Table 4 presents the univariable and multivariable analyses using the initial unmatched cohort and the logistic analysis from the propensity score-matched cohort, evaluating the effect on the diagnostic yield and complication risk of pneumothorax when ENB was used instead of R-EBUS. By adjusted analyses, using ENB over R-EBUS was associated with a higher overall diagnostic yield (OR=3.45, 95% CI=1.75–6.82 in the unmatched cohort, and OR=2.17, 95% CI=1.11–4.23 in the matched cohort) and higher sensitivity for malignancy (OR=7.14, 95% CI=3.20–15.92 in the unmatched cohort, and OR=3.55, 95% CI=1.75–7.22 in the matched cohort). Selecting either ENB or R-EBUS did not significantly affect the risk of pneumothorax. The regression analyses were conducted assuming all indeterminate cases as true negatives, and the full regression models are presented in the Supplementary Tables 2–5.
Predictions of outcomes for ENB versus R-EBUS.
| Outcome | OR | 95% CI | p-Value |
|---|---|---|---|
| Overall diagnostic yield | |||
| Unmatched, without adjustment | 2.08 | 1.21–3.59 | 0.008 |
| Unmatched, adjusted with multivariable analysisa | 3.45 | 1.75–6.82 | <0.001 |
| Propensity-score matched | 2.17 | 1.11–4.23 | 0.023 |
| Sensitivity for malignancy | |||
| Unmatched, without adjustment | 3.37 | 1.88–6.05 | <0.001 |
| Unmatched, adjusted with multivariable analysisb | 7.14 | 3.20–15.92 | <0.001 |
| Propensity-score matched | 3.55 | 1.75–7.22 | <0.001 |
| Complication of pneumothorax | |||
| Unmatched, without adjustment | 0.40 | 0.13–1.20 | 0.101 |
| Unmatched, adjusted with multivariable analysisc | 0.39 | 0.13–1.21 | 0.103 |
| Propensity-score matched | 0.41 | 0.10–1.64 | 0.208 |
ENB, electromagnetic navigation bronchoscopy; R-EBUS, radial endobronchial ultrasound; OR, odd ratio; CI, confidence interval.
To the best of our knowledge, this study represents the first patient-level evaluation to compare sole ENB-guided and sole R-EBUS-guided biopsy under moderate sedation for diagnosing pulmonary lesions. In a 1:1 propensity score-matched cohort constructed to achieve balance for pre-procedural factors, our study revealed a significantly higher diagnostic yield and sensitivity for malignancy for ENB than for R-EBUS. Sole ENB was superior to sole R-EBUS under moderate sedation for lesions>20mm in size, radiologically solid lesions, and lesions with a class 2 bronchus sign (an airway directly aligned with the lesion on CT scan). In the multivariable analysis using the unmatched cohort and the analysis after propensity score matching, using ENB over R-EBUS was significantly associated with a higher diagnostic yield. The complication rate was generally low (4.8% for pneumothorax) in this matched cohort, with no procedure-related respiratory failure or mortality events. No significant difference in the safety profiles was observed between the two techniques. Our study provides important insight on the utility of ENB over R-EBUS by directly comparing both techniques in a least-invasive setting from an Asian population, of which current data are insufficient.
Currently, guidelines on the diagnosis of lung lesions recommend that the type of diagnostic biopsy should be based on the location, size, invasiveness of the available procedure, and the possible risk of complications.8,9 Recent advances in image-assisted bronchoscopy technologies, such as ENB and R-EBUS, which demonstrated improved diagnostic yield and safety, have made transbronchial approach more feasible.25,26 To date, most studies on the utility and safety of ENB or R-EBUS have reported a diagnostic yield between 38% and 90%.16,25–28 The most recently reported meta-analyses on the diagnostic performance of these advanced bronchoscopic techniques have presented that for ENB, a pooled diagnostic yield of 76.4% and a sensitivity of 70.7–77% was achieved.15,18 For R-EBUS, a pooled diagnosed yield of 72.4% and a sensitivity of 70.5–72% were reported.17,18 However, significant variations on the performance patterns exist within and between studies in terms of case selection, concurrent use of both modalities, mixed use of additional complementary techniques, and type of anesthesia. This hampers the relevant evaluation and comparison between both techniques performed in a minimally invasive setting.
The NAVIGATE study, which is the largest multi-center study on ENB to date, has reported that among the 1388 participants who underwent ENB, 50.6% received concurrent R-EBUS guidance. Other complementary techniques included fluoroscopy in 85.0% and ROSE in 61.7% of the cases, and 78.2% received the procedure under general anesthesia.14 Similarly, a substantial proportion of published studies on the performance of R-EBUS presented results with additional complementary tools such as fluoroscopy, virtual bronchoscopy, and ROSE,29–32 without strict control for the type of sedation.33,34 The difficulty to control for and compare different bronchoscopy techniques in various settings is also noticeable from the results of the AQuIRE registry, which revealed significant variations on practice and sedation patterns for diagnostic bronchoscopy.26 The only available randomized controlled trial to compare the performance of sole ENB and R-EBUS at a patient level to date was reported by Eberhardt et al., which revealed an insignificant difference between the diagnostic yield of ENB (59%) and R-EBUS (69%). However, this study was not designed to strictly control for lesion characteristics and type of sedation.12 In this aspect, the design and results of our study are particularly relevant because there is no study comparing the diagnostic yield of sole ENB and R-EBUS matched for pre-procedural characteristics including radiologic findings. Moreover, in our study cohort, owing to the minimalized practice standards on guided bronchoscopy in the institute where the study was conducted, the use of additional technologies and the type of sedation was completely controlled. Our study also provides relevant data on the performance and safety of the sole procedures under moderate sedation without the use of complementary tools. Regarding data that suggest that general anesthesia can be associated with a greater need for post-procedure escalation of care in bronchoscopic procedures,35,36 sole procedure under moderate sedation would be the least-invasive setting for ENB and R-EBUS. Accordingly, our data would serve as a good reference across numerous clinical settings with no access to general anesthesia or other complementary techniques.
In our study, the diagnostic yield achieved by sole ENB was higher than the generally reported results from previous studies.18,27 Meanwhile, the yield of R-EBUS may be marginally lower.16,18,28 The relatively low diagnostic yield achieved by R-EBUS in our study could have been contributed by the infeasibility to use an aspiration needle for tissue sampling and complementary ROSE. Although transbronchial forceps biopsy remains the mainstream modality for tissue sampling for both ENB and R-EBUS,26,37,38 a recent updated meta-analysis on the diagnostic performance of R-EBUS has reported that the use of ROSE was associated with a higher sensitivity.17 However, ROSE may not solely impact the diagnostic value of R-EBUS as it is more likely to be used and associated with a higher diagnostic yield when transbronchial needle aspiration (TBNA) is performed;39 TBNA can also be a driving factor for increased performance of R-EBUS.26,40 By contrast, a recent updated meta-analysis on the diagnostic performance of ENB has revealed no significant difference with using complementary ROSE or using different type of biopsy tools.15 Likewise, our study revealed a similar diagnostic yield achieved by ENB-guided TBNA and transbronchial forceps biopsy.
In our study, after adjusting for potential factors (e.g. lesion size, location, and presence and the type of the bronchus sign) reported by single studies to affect the diagnostic yield of guided bronchoscopy,21 the use of ENB over R-EBUS was associated with a higher overall diagnostic yield and higher sensitivity for malignancy, without difference in the complication rates. This emphasizes the superior utility of ENB over R-EBUS when performed for similar pre-procedural settings. In most clinical settings, R-EBUS is more widely accessible than ENB since the expense of ENB consumables required per case is higher than R-EBUS. The rationale for using ENB over R-EBUS would be that the former may offer a higher diagnostic yield, as shown in our study.19 In addition, the higher proportion of those who underwent sequential linear EBUS for nodal evaluation in the ENB group than the R-EBUS group with similar total procedure time may indicate an additional benefit of shortening the total procedural time using ENB over R-EBUS. When selecting a sole modality under moderate sedation, as long as the financial cost for the health care facility is acceptable, using ENB would provide a better yield for diagnosing malignant lung lesions. Particularly, ENB would be recommended for solid lesions with a class 2 bronchus sign.
This study had some limitations. First, this was a retrospective study of patients from a single center, Therefore, the results may not be generalizable to other clinical settings, emphasizing the need for a well-designed prospective randomized controlled trial to corroborate our findings and apply them into future guidelines. Although we performed propensity score matching, an uncontrollable selection bias might have remained (e.g. the attending physician's preference for a particular situation). Second, our results may not validate the utility of sole ENB or R-EBUS under moderate sedation over other performance patterns in different clinical settings. Although the combination of ENB and R-EBUS and the combination of various tissue sampling techniques (e.g. transbronchial biopsy and TBNA) may improve the diagnostic yield, the current data on the efficacy of combining technologies and biopsy tools remain inconsistent. Moreover, concerns regarding financial burden increase with the combined use of various techniques, resulting in clinical settings with limited accessible modalities. Third, the number of lesions ≤20mm included in our study was relatively small. Therefore, the power to detect a statistical significance for the difference in the diagnostic performance between ENB and R-EBUS for smaller lesions may have been insufficient. In addition, a substantial proportion of lesions larger>30mm was included in our study. The relatively larger size of the included lesions could have possibly contributed to the better diagnostic performance of ENB. Nevertheless, our study provides valuable information on the superiority of sole ENB over R-EBUS in a least-invasive setting, and data on the mainstream modality with the least combination of complementary techniques. This would be the clinical setting that a maximal number of physicians would be accessible.
In conclusion, from a propensity score-matched analysis controlled for pre-procedural factors, sole ENB demonstrated a higher diagnostic yield than sole R-EBUS under moderate sedation for diagnosing pulmonary lesions suspicious for lung cancer, with similar and generally low complication rates. Our data support the utility of ENB over R-EBUS in a least-invasive setting and serve as a good reference for the choice of modality in similar clinical settings which would be accessible to a maximum number of physicians performing guided-bronchoscopy.
Author's contributionsYWK had full access to all of the study data and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. YWK, HJK, and SHY contributed to the concept and design of the study, data collection, data analysis, data interpretation, manuscript preparation, revision, and final approval of the manuscript. MJS, BSK, SYL, YJL, JSP, YJC, JHL, and CTL contributed to the data collection, data analysis, revision, and final approval of the manuscript.
Conflict of interestThe authors declare no conflict of interest.
This study was supported by the Seoul National University Bundang Hospital (grant number: 06-2022-0291). The authors thank the Division of Statistics in the Medical Research Collaborating Center of Seoul National University Bundang Hospital for assistance in statistical analyses.