We aimed to describe the effectiveness and safety of inhaled antibiotics in chronic obstructive pulmonary disease (COPD) patients, as well as the patient profile in which they are usually prescribed and the patient groups that can most benefit from this treatment.
MethodsMulticentre retrospective observational cohort study in COPD patients who had received ≥1 dose of inhaled antibiotics in the last 5 years. Clinical data from the two years prior to and subsequent to the start of the treatment were compared. Primary outcome: COPD exacerbations. Secondary outcomes: side effects, symptomatology (sputum purulence, dyspnoea), microbiological profile and pathogen eradication.
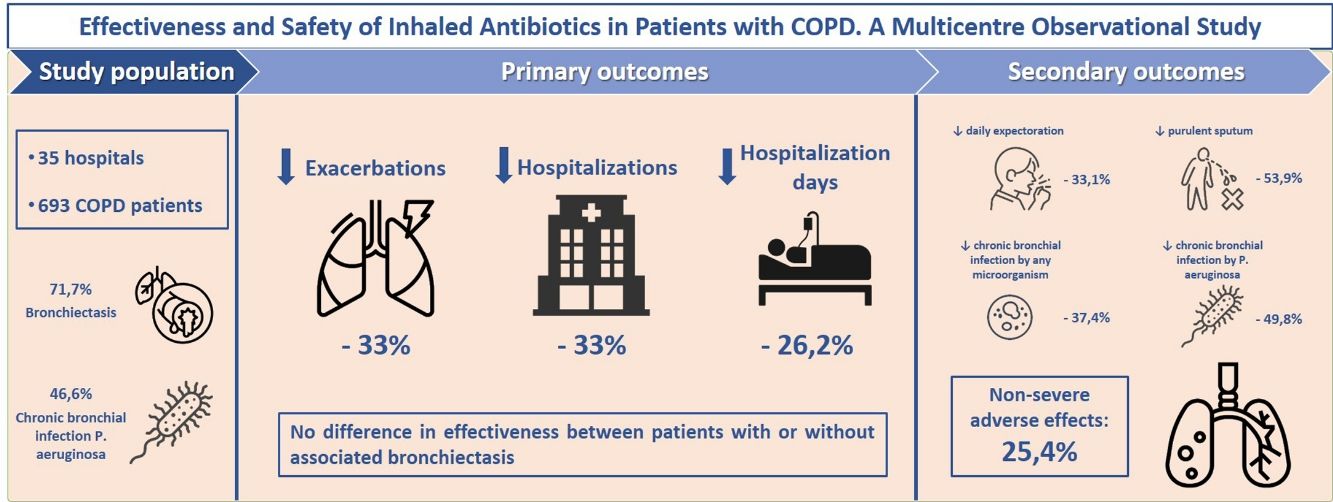
ResultsOf 693 COPD patients analyzed (aged 74.1; 86.3% men; mean FEV1=43.7%), 71.7% had bronchiectasis and 46.6% presented chronic bronchial infection (CBI) by Pseudomonas aeruginosa (PA). After 1 year of treatment with inhaled antibiotics, there was a significant decrease in the number of exacerbations (−33.3%; P<.001), hospital admissions (−33.3%; P<.001) and hospitalization days (−26.2%; P=.003). We found no difference in effectiveness between patients with or without associated bronchiectasis. Positive patient outcomes were more pronounced in PA-eradicated patients. We found a significant reduction in daily expectoration (−33.1%; P=.024), mucopurulent/purulent sputum (−53.9%; P<.001), isolation of any potentially pathogenic microorganisms (PPM) (−16.7%; P<.001), CBI by any PPM (−37.4%; P<.001) and CBI by PA (−49.8%; P<.001). CBI by any PPM and ≥three previous exacerbations were associated with a better treatment response. 25.4% of patients presented non-severe side-effects, the most frequent of these being bronchospasm (10.5%), dyspnoea (8.8%) and cough (1.7%).
ConclusionsIn COPD patients with multiple exacerbations and/or CBI by any PPM (especially PA), inhaled antibiotics appear to be an effective and safe treatment, regardless of the presence of bronchiectasis.
Nuestro objetivo fue describir la efectividad y seguridad de los antibióticos inhalados en enfermedad pulmonar obstructiva crónica (EPOC), así como el perfil de pacientes en los que se prescriben habitualmente y los grupos de pacientes que más pueden beneficiarse de este tratamiento.
MétodosEstudio de cohorte observacional retrospectivo multicéntrico en pacientes con EPOC que habían recibido ≥1 dosis de antibióticos inhalados en los últimos 5 años. Se compararon los datos clínicos de los 2 años anteriores y posteriores al inicio del tratamiento. Criterio primario: exacerbaciones de EPOC. Criterios secundarios: efectos secundarios, sintomatología (purulencia del esputo, disnea), perfil microbiológico y erradicación de patógenos.
ResultadosDe los 693 pacientes con EPOC analizados (74,1 años; 86,3% hombres; FEV1 medio=43,7%) el 71,7% presentaba bronquiectasias y el 46,6% presentaba infección bronquial crónica (IBC) por Pseudomonas aeruginosa (PA). Después de un año de tratamiento con antibióticos inhalados se produjo una disminución significativa en el número de exacerbaciones (−33,3%; p<0,001), ingresos hospitalarios (−33,3%; p<0,001) y días de hospitalización (−26,2%; p=0,003). No encontramos diferencias en la efectividad entre los pacientes con o sin bronquiectasias asociadas. Los resultados positivos fueron más pronunciados en los pacientes que erradicaron la PA. Encontramos una reducción significativa de la expectoración diaria (−33,1%; p=0,024), el esputo mucopurulento/purulento (−53,9%; p<0,001), el aislamiento de cualquier microorganismo potencialmente patógeno (MPP) (−16,7%; p<0,001), IBC por cualquier MPP (−37,4%; p<0,001) e ICB por PA (−49,8%; p<0,001). La IBC por cualquier MPP y más de 3 exacerbaciones previas se asociaron con una mejor respuesta al tratamiento. El 25,4% de los pacientes presentó efectos secundarios no graves, siendo los más frecuentes el broncoespasmo (10,5%), la disnea (8,8%) y la tos (1,7%).
ConclusionesEn los pacientes con EPOC con múltiples exacerbaciones o IBC por cualquier MPP (especialmente PA), los antibióticos inhalados parecen ser un tratamiento eficaz y seguro, independientemente de la presencia de bronquiectasias.
Up to half of patients with stable chronic obstructive pulmonary disease (COPD) may present with bronchial infection by potentially pathogenic microorganisms (PPM)1,2 during the course of the disease. This chronic infection is associated with increased bronchial and systemic inflammation,3,4 a higher number and greater severity of COPD exacerbations,5,6 accelerated loss of lung function7 and greater clinical severity,8 which in its turn significantly affects patients’ quality of life.9
Although Haemophilus influenzae (HI) represents the most common PPM isolated from the airways of these patients, the most severe cases of the disease are characterized by a higher prevalence of non-fermenting Gram-negative bacilli, in particular Pseudomonas aeruginosa (PA).10 Moreover, some studies suggest that infection with PA in patients with COPD may be associated with a poorer prognosis compared to patients whose airways have never been colonized by the pathogen.11
The use of inhaled antibiotics is recommended by several clinical practice guidelines on bronchiectasis and cystic fibrosis for these PPM (and others), especially in patients with chronic bronchial infection (CBI) due to PA.12–15 Furthermore, inhalation is an advantageous alternative to other routes of administration, since higher concentrations of the drug can be achieved in the airways with minimal systemic side-effects.16 Similarly, there is some evidence on the effectiveness and safety of inhaled antibiotics in COPD patients with frequent exacerbations and CBI.17–20 Increasing this evidence with larger observational studies may encourage the implementation of randomized clinical trials, which could lead to the establishment of firm guidelines in the future.21,22
In this regard, we have conducted a multicentre observational study addressing the risk–benefit relationship of inhaled antibiotics and the extent to which they are used in patients with COPD. The specific aims of the study were to describe the patient population and the indications for which these drugs are prescribed, analyze the effectiveness and safety of this treatment in COPD patients and in clinically relevant subgroups of patients and identify the variables associated with a better clinical response.
MethodsStudy Design and PopulationWe carried out a multicentre observational study of historical cohorts of COPD patients. Inclusion criteria were: age >35 years, smoking habits >10 pack-years, post-bronchodilator forced expiratory volume in 1s (FEV1)/forced vital capacity (FVC) <0.7, and the administration of least one dose of any inhaled antibiotic in the five years prior to their inclusion in the study. We did not establish any exclusion criteria, but for the effectiveness analysis, only those patients for whom exacerbation data were available for at least 1 year were included.
All members of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) were informed of the details of the study and were invited to take part in it, by means of an e-mail communication. Those centres interested in participating contributed applicable cases, but no other pre-determined criteria were used to select them. The study was approved by the Clinical Research Ethics Committee of Catalonia (number: CEI 18/94). Informed consent was not requested as information obtained from the medical history was dissociated from the personal identification data, in accordance with the current data protection law (Regulation (EU) No. 2016/679 of the European Parliament and of the Council of 27 April 2016 on Data Protection). The study follows the STROBE guidelines and conforms to the principles outlined in the Helsinki Declaration.
Data CollectionPatients who had received at least one dose of any inhaled antibiotic between 2013 and 2018 were identified using the hospital pharmacy records. This list was cross-referenced to information obtained from medical records on the diagnosis of COPD. Data were collected by the end of 2019, so that there would be at least 1 year of follow-up in all patients after starting to take inhaled antibiotics.
The data were collected via a standardized protocol. They included exacerbations, spirometry, radiological and microbiological data and treatment with inhaled antibiotics (active substance, dose, duration and adverse effects). Comorbidities were calculated by the Charlson comorbidity index.23
Outcome Measures and Description of the VariablesThe primary outcome of the study was the difference in the mean annual COPD exacerbation rate in the year before and the year after starting inhaled antibiotics. COPD exacerbations were defined as acute episodes of sustained worsening of respiratory symptoms that required outpatient pharmacological treatment or hospital admission.21 Secondary outcomes were COPD symptoms (expectoration, sputum purulence and dyspnoea), microbiological profile and PPM eradication from sputum.
Sputum staining was analyzed following the validated scale of Murray et al.24 Bacteria isolated in sputum cultures were classified as PPM strains in the case of HI, Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), PA and other Gram-negative bacilli.
We defined CBI as the presence of at least three positive sputum cultures for the same PPM in a 12-month period, one month apart.25 Conversely, patients were considered free from chronic infection when at least three consecutive cultures were negative for the same PPM in a 12-month period, one month apart.25 Bronchiectasis were diagnosed by high-resolution computed tomography (HRCT).12
Statistical AnalysisFor both the descriptive and safety analysis, data from all the patients who had received at least one dose of inhaled antibiotic in the last five years were included. For the effectiveness analysis (primary and secondary outcomes), we only considered patients with a known number of exacerbations that appeared at least 1 year after the start of treatment (≥1 year of follow-up data). Moreover, a sensitivity analysis was carried out in those patients who used inhaled antibiotics for a minimum of 3 months after their prescription and in those who had bronchiectasis confirmed by HRCT.
Quantitative variables were expressed by the mean and standard deviation or the median and interquartile range, depending on whether they followed a normal distribution determined by the Kolmogorov–Smirnov test. If so, the Student's t-test was used for independent or paired means, while the Mann–Whitney or Wilcoxon U tests were applied to variables with non-normal distribution, respectively. Either the ANOVA or Kruskal–Wallis tests were used to compare more than two means or medians, depending on the specific case. Qualitative variables were expressed according to their relative percentage and compared with the Chi-square and Fisher's exact tests.
Variables associated with annual changes in the number of exacerbations before and after inhaled antibiotic treatment were assessed using a mixed-effects linear regression model allowing for random intercepts. The variables introduced into the multivariate model were selected on the basis of their clinical relevance: age, gender, presence of bronchiectasis, CBI by any PPM, post-bronchodilator FEV1 (%predicted), smoking habit and annual number of exacerbations in the two years prior to treatment with inhaled antibiotics.10 Statistical significance was defined by P<.05. The IBM® Statistical Package for the Social Sciences (SPSS®) software version 24 was used.
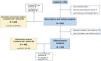
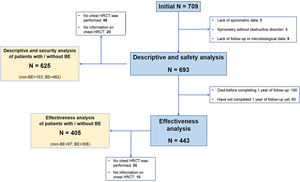
ResultsStudy Population and TreatmentA total number of 709 patients from 35 hospitals throughout Spain were considered for the study. Of these, 693 (97.7%) met all the inclusion criteria for the descriptive and safety analysis (Fig. 1). Table 1 shows the baseline characteristics of the patients when inhaled antibiotics were prescribed. The mean age was 74.1 (standard deviation (SD)=8.5) years, and 86.3% of the study population were men. The mean FEV1 was 43.7% (SD=16.9%). Most of the patients presented with daily expectoration (76.9%), mainly mucopurulent (48%), while 94.4% and 88.9% were under treatment with long-acting beta-adrenoceptor agonist (LABA) or long-acting muscarinic antagonist (LAMA) bronchodilators, respectively, and 83.7%, with inhaled corticosteroids.
Baseline Sociodemographic and Clinical Characteristics of the Study Population (Non-stratified and Stratified Data by Absence or Presence of Bronchiectasis According to Thoracic HRCT). Significant Results are Indicated in Bold (P<.05).
| Total(N=693) | Non-bronchiectasis(N=163)a | Bronchiectasis(N=462) a | Non-bronchiectasis vs Bronchiectasis (P Value) | Missing Values (N) | |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Gender (% of men) | 86.3 | 88.3 | 85.5 | .360 | – |
| Age at the start of IA, years (mean±SD) | 74.1±8.5 | 73.7±8.1 | 73.9±8.4 | .559 | – |
| Smoking habit, packs/year (mean±SD) | 51.1±27.0 | 55.4±29.7 | 49.9±26.3 | .037 | – |
| Currently smoking (% of patients) | 5.8 | 8 | 4.5 | .098 | – |
| Charlson comorbidity index, points (mean±SD) | 5.46±2.3 | 5.38±2.4 | 5.37±2.3 | .256 | 71 |
| Symptoms | |||||
| Daily expectoration (% of patients) | 76.9 | 65.4 | 82.6 | <.001 | 21 |
| Type of expectoration (% of patients) | |||||
| White | 24.9 | 34.6 | 21.7 | ||
| Mucopurulent | 48 | 40.4 | 49.6 | .035 | 70 |
| Purulent | 25.8 | 22.8 | 27.4 | ||
| Degree of dyspnoea (mMRC) (mean±SD) | 2.46±0.89 | 2.44±0.82 | 2.43±0.91 | .066 | 51 |
| Respiratory function | |||||
| FVC (mean %pred±SD) | 68.1±20.3 | 67.1±16.9 | 68.8±20.8 | .003 | 7 |
| FEV1(mean %pred±SD) | 43.7±16.9 | 42.7±14.7 | 44.5±17.5 | .042 | 5 |
| FEV1/FVC (mean±SD) | 47.6±12.1 | 46.9±11.9 | 48.2±12.0 | .764 | 2 |
| SaO2(mean %±SD) | 92.6±4.2 | 92.5±3.8 | 92.6±4.4 | .558 | 124 |
| Radiological data | |||||
| Presence of bronchiectasis (% of patients) | 71.7 | 0 | 100 | – | –a |
| Lobules with bronchiectasis, No. (mean±SD) | 2.7±1.2 | 0 | 2.7±1.2 | – | 201a |
| Presence of emphysema (% of patients) | 65.8 | 66.3 | 65.7 | .898 | 13a |
| Microbiological data (% of patients) | |||||
| Isolation of Pseudomonas aeruginosa | 91.6 | 89.6 | 91.3 | .433 | – |
| Isolation of Haemophilus influenzae | 15.3 | 22.1 | 12.1 | .002 | – |
| Isolation of Stenotrophomonas maltophila | 13 | 17.8 | 11.3 | .033 | – |
| Isolation of Escherichia coli | 6.9 | 6.7 | 7.6 | .728 | – |
| Isolation of Staphylococcus aureus | 6.2 | 6.1 | 6.3 | .949 | – |
| Isolation of Streptococcus pneumoniae | 5.6 | 7.4 | 5.6 | .426 | – |
| Isolation of Moraxella catarrhalis | 5.5 | 7.4 | 5.2 | .307 | – |
| Isolation of Aspergillus spp. | 5.5 | 8 | 5.2 | .196 | – |
| Isolation of Achromobacter xylosoxidans | 3.9 | 3.7 | 3.9 | .902 | – |
| Isolation of MRSA | 2.2 | 5.5 | 1.1 | .001 | – |
| CBI by P. aeruginosa | 46.6 | 31.9 | 51.5 | <.001 | – |
| CBI by any PPM | 51.5 | 36.2 | 57.1 | <.001 | – |
| Exacerbations during the 2 years before starting the treatment with IA | |||||
| Exacerbations per year, No. (mean±SD) | 2.9±2.1 | 3.0±2.5 | 2.8±1.9 | .405 | – |
| Hospital admissions per year, No. (mean±SD) | 1.3±1.3 | 1.3±1.3 | 1.2±1.3 | .416 | – |
| Days of hospitalization, No. (mean±SD) | 13.6±16.5 | 13.8±15.6 | 13.4±16.2 | .750 | 1 |
| Patients with exacerbator phenotype in each of the 2 years (% of patients) | 84.5 | 84.7 | 84.4 | .940 | – |
| Treatments (% of patients) | |||||
| LABA | 94.4 | 97.5 | 94.4 | .260 | – |
| LAMA | 88.9 | 90.8 | 87.9 | .808 | – |
| ICS | 83.7 | 86.5 | 83.3 | .336 | – |
| Long-term azithromycin | 36.8 | 34.4 | 37.5 | .470 | – |
| Roflumilast | 10.4 | 9.8 | 10.2 | .890 | – |
| Long-term mucolytics | 30.6 | 23.3 | 33 | .021 | – |
| Long-term oral corticosteroids | 14.6 | 15.3 | 11.7 | .232 | – |
| Oral cyclic antibiotics | 6.5 | 2.5 | 8.2 | .011 | – |
| Long-term oxygen therapy | 51.2 | 57.7 | 48.4 | .041 | – |
| Pulmonary rehabilitation programme | 35.9 | 31.9 | 36.2 | .320 | – |
CBI: chronic bronchial infection; HRCT: high-resolution computed tomography; FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; IA: inhaled antibiotics; ICS: inhaled corticosteroids; LABA: long-acting beta-adrenoceptor agonist; LAMA: long-acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale; MRSA: methicillin-resistant Staphylococcus aureus; PPM: potentially pathogenic microorganisms; SaO2: oxygen saturation; SD: standard deviation; %pred: predicted percentage.
The median number of sputum cultures collected per patient during the two years prior to the start of inhaled antibiotics was 6 (IQR=4–9) (range=1–54). The median time elapsed between the first positive sputum and the start of the treatment was 10.1 months (IQR=3.7–19) (range=0–84). At least one PPM was isolated in 100% of the patients, with PA being the most frequently involved (91.6%); 51.5% of patients met the criteria for CBI by any PPM, with PA again being the most frequent (Table 1). The main reason for starting inhaled antibiotics was the presence of frequent exacerbations in patients with positive cultures, regardless of the presence of criteria for CBI. In fact, when patients with and without these criteria were compared, there was no significant difference in the number of exacerbations (3.5 vs. 3.1; P=.202) or hospitalizations (1.7 vs. 1.5; P=.062) during the previous year.
Patients were given colistimethate sodium (82.5%), tobramycin (15.3%), ceftazidime (1.6%), gentamicin (0.4%) or amikacin (0.1%) (e-Table 1) for a median time of 8.9 (interquartile range (IQR)=3.3–22) months (range=0.1–71.5).
When the patients for whom HRCT information was available (625 patients; 90.2%) were considered separately, we found that 462 (73.9%) of them had bronchiectasis (Fig. 1). Compared with patients without bronchiectasis, these patients presented with increased daily expectoration (P<.001), more frequent mucopurulent or purulent sputum (P=.035), a higher proportion of CBI by PA or any PPM (both P<.001) and an increased use of long-term mucolytics (P=.021) and oral cyclic antibiotics (P=.011) (Table 1).
Effectiveness on COPD Exacerbation RatesThe exclusion of patients with insufficient follow-up data left a subtotal of 443 (63.9%) individuals for analysis (Fig. 1). e-Table 2 shows the differences between these patients and the remaining population.
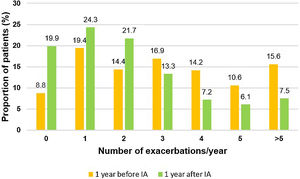
During the two years prior to starting inhaled antibiotics, the mean number of exacerbations and hospital admissions progressively increased, as well as the proportion of patients with exacerbator phenotype (Fig. 2). However, when comparing the first year prior to and the first year subsequent to the start of inhaled antibiotics (primary outcome), we observed a significant decrease in the mean number of exacerbations (−33.3%; P<.001) (Table 2 and Fig. 2), mainly due to the lower percentage of patients with three or more events after the treatment (Fig. 3). The number of hospital admissions and days of hospitalization also dropped (−33.3%; P<.001 and −26.2%; P=.003, respectively) (Table 2 and Fig. 2). When the analysis was extended to a two-year period before and after antibiotics, the reduction of exacerbations (−27.6%; P<.001) and hospitalizations (−25%; P=.002) was still significant (Table 2 and Fig. 2). Moreover, the significant decrease in the percentage of patients with exacerbator phenotype was observed up to two years after starting the treatment (Fig. 2). These findings were maintained when patients (N=387; 55.8%) who received inhaled antibiotics for at least 3 months were considered (e-Figure 1).
Evolution of patients with COPD under treatment with inhaled antibiotics: (A) number of exacerbations and hospital admissions and (B) proportion of patients with exacerbator phenotype. N=443. IA: inhaled antibiotics. §=P<.001 (compared to the year before IA); #=P=.42 (compared to two years before IA); ¥=P<.001 (compared to two years before IA).
Comparative Analysis of COPD Exacerbations (Primary Outcome) Before and After Inhaled Antibiotics in Non-stratified and Stratified Patient Data According to Absence or Presence of Bronchiectasis. Significant Results Are Indicated in Bold (P<.05).
| Total | Non-bronchiectasisa | Bronchiectasisa | Bronchiectasis vs. Non-bronchiectasis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Years Before IA(n=345) | 2 Years After IA(n=345) | Change (%);Intragroup P Value | 2 Years Before IA(n=83) | 2 Years After IA(n=83) | Change (%);Intragroup P Value | 2 Years Before IA(n=262) | 2 years after IA(n=262) | Change (%);intragroup p value | Change (%); Intergroup P Value | |
| Exacerbations, No. (mean±SD) | 2.9±2.2 | 2.1±1.8 | −27.6; <.001 | 3.3±2.8 | 2.4±2.0 | −27.3; <.001 | 2.8±1.9 | 2.0±1.7 | −28.6; <0.001 | .970 |
| Hospital admissions, No. (mean±SD) | 1.2±1.2 | 0.9±1.2 | −25; .002 | 1.2±1.2 | 1.0±1.2 | −16.7; .111 | 1.1±1.2 | 0.9±1.2 | −18.2; 0.004 | .524 |
| Hospitalization, days (mean±SD) | 11.9±14.7 | 11.6±23.1 | −2.5; .790 | 11.5±12.3 | 11.1±17.4 | −3.5; .788 | 12.1±15.3 | 11.8±24.7 | −2.5; 0.882 | .251 |
| Total | Non-bronchiectasisa | Bronchiectasisa | Bronchiectasis vs. Non-bronchiectasis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Year Before IA(n=392) | 1 Year After IA(n=392) | Change (%);Intragroup P Value | 1 Year Before IA(n=92) | 1 Year After IA(n=92) | Change (%);Intragroup P Value | 1 Year Before IA(n=300) | 1 Year After IA(n=300) | Change (%);Intragroup P Value | Change (%); Intergroup P Value | |
| Exacerbations, No. (mean±SD) | 3.3±2.5 | 2.2±2.1 | −33.3; <.001 | 3.8±3.0 | 2.4±2.3 | −36.8; <.001 | 3.2±2.3 | 2.1±2.0 | −34.4; <.001 | .911 |
| Hospital admissions, No. (mean±SD) | 1.5±1.7 | 1.0±1.5 | −33.3; <.001 | 1.5±1.6 | 1.0±1.4 | −33.3; .003 | 1.5±1.7 | 1.0±1.4 | −33.3; <.001 | .846 |
| Hospitalization, days (mean±SD) | 16.0±22.6 | 11.8±25.0 | −26.2; .003 | 16.5±20.4 | 10.6±18.3 | −35.7; .003 | 15.8±23.1 | 12.2±26.8 | −22.8; .061 | .785 |
IA: inhaled antibiotics; SD: standard deviation.
Twenty-one patients (4.7%) received treatment for less than 1 month, mainly due to side-effects that led to discontinuation (in 17 cases). In these patients, the mean number of exacerbations, hospitalizations and days of hospitalization in the subsequent year was significantly higher than in those who completed more than 1 month of treatment.
The multiple linear regression analysis (Table 3) identified independent factors associated with a significant change in the number of exacerbations. CBI by any PPM (beta coefficient=−0.22; 95% confidence interval (CI)=−0.31, −0.55; P<.001) and at least three exacerbations in each of the previous two years (beta coefficient=−0.18; 95% CI=−1.3, −0.42; P<.001) were associated with a significant decrease in the number of exacerbations. Finally, since the vast majority of CBI seen in our patients were by PA, we performed an additional multivariable analysis changing CBI by any PPM for CBI by PA, and the results did not change (beta coefficient: −0.25; 95% CI: −0.34, −0.59; P<.001).
Factor Independently Associated With Statistically Significant Changes in the Number of Exacerbations.
| Variables | Beta Coefficient | P Value | 95% CI |
|---|---|---|---|
| CBI by any PPM | −0.22 | <.001 | −0.31 to −0.55 |
| At least 3 exacerbation per year (two previous years) | −0.18 | <.001 | −0.42 to −1.33 |
Adjusted by age, gender, presence of bronchiectasis, post bronchodilator FEV1 (% predicted), and smoking habit.
Regarding secondary outcomes, inhaled antibiotics were associated with a significant reduction in daily expectoration (80.8% vs. 55.7% of patients; P<.024) and mucopurulent or purulent sputum (73% vs. 36.9%; P<.001) after one year of treatment, although spirometry performance slightly worsened after one year of treatment (69.4% vs. 67.7% of mean predicted FVC and 44.8% vs. 43.7% of mean predicted FEV1) (Table 4).
Effectiveness of Inhaled Antibiotics on COPD Symptoms, Respiratory Function and Microbiological Profile (Secondary Outcomes). Data Comprising One Year Before and One Year After Treatment Onset Are shown. Significant Results Are Indicated in Bold (P<.05). N=443.
| Before IA | After IA | P Value | Missing Values (N) | |
|---|---|---|---|---|
| Symptoms (% of patients) | ||||
| Daily expectoration | 80.8 | 55.7 | .024 | 24 |
| White expectoration | 25.5 | 62.2 | <.001 | 89 |
| Mucopurulent/purulent expectoration | 73 | 36.9 | <.001 | 89 |
| Respiratory function | ||||
| FVC (mean %pred±SD) | 69.4±20.6 | 67.7±20.3 | .014 | 124 |
| FEV1 (mean %pred±SD) | 44.8±17.7 | 43.7±18.4 | .002 | 121 |
| FEV1/FVC (mean±SD) | 47.8±12.0 | 47.8±13.0 | .409 | 126 |
| Microbiologicaldataa(% of patients) | ||||
| Isolation of Pseudomonas aeruginosa | 91.2 | 54 | <.001 | – |
| CBI by P. aeruginosa | 49.4 | 24.8 | <.001 | – |
| Eradication of P. aeruginosa | – | 38.8 | – | – |
| Isolation of Haemophilus influenzae | 15.1 | 6.3 | .015 | – |
| CBI by H. influenzae | 0.9 | 0.9 | 1.0 | – |
| Isolation of Stenotrophomonas maltophila | 12.9 | 10.2 | .158 | – |
| CBI by S. maltophila | 1.6 | 0.9 | .318 | – |
| Isolation of any PPM | 100 | 83.3 | <.001 | – |
| CBI by any PPM | 54.9 | 34.3 | <.001 | – |
CBI: chronic bronchial infection; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; IA: inhaled antibiotics; PPM: potentially pathogenic microorganism; SD: standard deviation; %pred: predicted percentage.
The microbiological profiles of the sputum samples show that PA and HI were isolated to a lesser extent (91.2% of patients vs. 54%; P<.001 and 15.1% vs. 6.3%; P=.015, respectively) (Table 4 and e-Figure 2). We also found a reduction in the prevalence of CBI by PA (49.4% vs. 24.8%; P<.001), while PA was eradicated in 38.8% of COPD cases (Table 4).
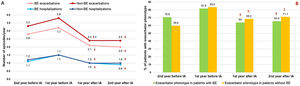
Inhaled Antibiotics in Clinically Relevant Subgroups of PatientsWe sought to further analyze the effectiveness of inhaled antibiotics depending on the presence or absence of bronchiectasis (Fig. 1). After stratifying the data, we did not find any relevant differences in treatment effectiveness after one year. Both subgroups of patients separately showed a significant reduction in the number of exacerbations (−34.4% in patients with bronchiectasis; P<.001 and −36.8% in patients without bronchiectasis; P<.001), admissions (−33.3%; P<.001 and −33.3%; P=.003) and days spent in hospital (−22.8%; P=.061 and −35.7%; P=.003) (Table 2). Clinical improvement continued until two years after the initiation of inhaled antibiotics in both these subgroups of COPD patients (Fig. 4).
Evolution of patients with and without bronchiectasis before and after the start of inhaled antibiotic treatment: (A) number of exacerbations (upper graph) and hospital admissions (lower graph) and (B) proportion of patients with exacerbator phenotype. N=405. BE: bronchiectasis; IA: inhaled antibiotics. §=P<.001; ¥=P=.003; €=P=.015; ∏=P=.002; ∑=P=.04 (compared to the year before IA); #=P=.015 (compared to two years before IA).
We found that PA eradication was achieved in a significantly higher proportion of patients with no criteria for CBI by PA, compared to those patients who already had CBI by PA (49.2% vs. 37.0%; P=.013). Furthermore, we found that the effectiveness of the treatment varied according to the achievement of eradication of airway PA. PA-free patients presented less exacerbations (1.7; P<.001 vs. 2.2; P<.001) and hospitalizations (0.7; P<.001 vs. 1.0; P<.001) per year compared with patients with persistent PA-positive cultures (Fig. 5). There was a smaller percentage of deaths (23.3% vs. 32.3%; P=.046) and exacerbator phenotype (61%; P<.001 vs. 71.4%; P=.007) after 2 years of follow-up when PA was successfully eradicated (e-Table 3 and Fig. 5). Additional sociodemographic and clinical baseline features of both subgroups of patients may be checked on e-Table 3.
Evolution of patients with or without successful eradication of PA (sputum isolates or CBI) before and after the start of inhaled antibiotic treatment: (A) number of exacerbations and hospital admissions and (B) proportion of patients with exacerbator phenotype. N=404. IA: inhaled antibiotics; PA: Pseudomonas aeruginosa. §=P<.001; €=P=.015; £=P=.006; ∏=P=.001; ∑=P=.007 (compared to the year before IA); #=P=.009; ¥=P<.001 (compared to two years before IA).
A total of 176 patients (25.4%) suffered from adverse events related to treatment with inhaled antibiotics after a median time frame of 22.5 days (IQR=7.7–90). The most frequent side-effects were bronchospasms (10.5%), dyspnoea (8.8%) and cough (1.7%) (e-Table 4). Although none were classified as serious, medication had to be changed in 6.2% of patients and discontinued in another 15.9% after 56 days (IQR=17–122), mainly due to dyspnoea or cough (82% of cases).
DiscussionThe use of inhaled antibiotics was associated with a significant reduction in the number and severity of COPD exacerbations for at least one year, regardless of the presence of bronchiectasis. This treatment was also effective in terms of reductions in daily expectoration, sputum purulence, PPM (HI and PA) isolation and CBI by PA. The latter was successfully eradicated in a large proportion of patients, so the effect of inhaled antibiotics was especially favourable in these cases. Other variables related to a better clinical response to inhaled antibiotics were CBI by any PPM and at least three previous exacerbations. None of the observed side effects was classified as severe, and bronchospasm was the most frequent.
Currently, there are no indications for the use of inhaled antibiotics in COPD, despite the known deleterious effects of CBI in these patients.6–9,11 The few small observational studies that have addressed this question so far have reported an improvement in inflammatory parameters and decreased bacterial load, exacerbations and hospitalizations, with few serious adverse effects.17–19 These results have been reproduced and extended in the multicentre study above, but with a much larger sample of patients, confirming the clinical effect of inhaled antibiotics on the reduction of COPD exacerbations. As in the aforementioned studies, we also found a clear male predominance, a high proportion of bronchiectasis18,19 and a follow-up time similar to that of the longest study.19 In contrast to the previous studies in which CBI was present in all cases, some patients in our study were included without CBI by PA. Moreover, the patients included here met most of the criteria for COPD severity – namely, severe impairment in airflow obstruction, frequent cough and expectoration, PA presence, exacerbator phenotype, presence of bronchiectasis and a high pharmacological burden, including inhaled bronchodilators and steroids, azithromycin and oxygen therapy.
Of the clinical variables, CBI by any PPM (specially by PA) and/or multiple exacerbations were correlated with better treatment outcomes in our study, although they are usually related to poorer patient prognosis.11,26 Thus, the mechanisms underlying the general effectiveness of treatment with inhaled antibiotics in severe COPD patients may include a decreased bacterial load in the airway and the local and systemic inflammation associated with it. Furthermore, the reduction in exacerbations with inhaled antibiotics was >20%, which coincides with the minimum difference in exacerbation rates arbitrarily suggested for use in COPD trials,27 underscoring the pivotal role of bronchial infection in COPD progression.
Another striking finding of our study that further supports the clinical relevance of PPM in the lungs of COPD patients was the larger reduction in exacerbation and hospitalization rates when PA eradication was achieved. These favourable results of inhaled antibiotic treatment in primary infection or CBI due to PA should be confirmed in placebo-controlled trials specifically designed for this purpose.
Interestingly, the presence of bronchiectasis in COPD patients did not alter the significance of treatment effectiveness. In fact, although bronchiectasis in COPD is associated with a higher frequency of PPM isolates and CBI,28 some patients present with CBI by PA independently of these.29 Also, a recent work by Martínez-García et al. identifies sputum-derived PPM positive cultures as bronchiectasis risk factors, and not the other way round.30 Taken together, and in the current absence of randomized controlled trials, these observations may explain why inhaled antibiotics should be considered on the basis of the respiratory microbiological profile (especially CBI) and frequent COPD exacerbations, rather than abnormalities in radiological data.31,32 Furthermore, early treatment of CBI may prevent or delay the development of bronchiectasis and thus improve patients’ prognosis.30
Adverse effects were reported in 25% of patients, of which bronchospasms were the most frequent and none were serious. Treatment had to be discontinued in 15.9% of cases. These percentages diverge from previous studies,18,19 possibly due to: the inclusion of patients who received at least one dose of inhaled antibiotic and not only patients receiving long-term treatment; the consideration of patients who started inhaled antibiotics during an exacerbation; and a certain degree of heterogeneity in the treatment initiation and follow-up criteria in the participating centres. In any case, it is advisable to monitor the appearance of any side-effects.
A limitation of this work may be a lack of control over possible unknown or unmeasured confounding variables, given its retrospective nature, although it may better characterize daily clinical practice regarding the use of inhaled antibiotics. In this regard, it should be noted that we could not obtain detailed and reliable information about the aetiology of exacerbations, nor did we collect data on treatment or spirometric evolution over the previous two years. We did not collect data on the modification during the follow-up time of certain treatments that patients were taking at baseline (azithromycin, roflumilast), which might have influenced the results in some way. Patients who were not included due to insufficient follow-up time were more severe and had worse outcomes. Since our main objective was to investigate the effectiveness of inhaled antibiotics in the reduction of exacerbation, we could only analyze patients with at least one year of follow-up, so patients with end-stage disease were not evaluated. Worse outcomes may be exclusively due to more severe baseline disease, so that the possible conclusion that inhaled antibiotics might be less effective in end-stage disease would require a placebo-controlled trial to confirm it. Similarly, given that there were only 97 individuals in the subgroup of patients without bronchiectasis, we cannot rule out the occurrence of a type II error in non-significant comparisons, although this does not apply to the comparisons that were statistically significant. Moreover, despite the fact that no data on antibiotic sensitivity were collected, no increase was detected in the proportion of intrinsically resistant PPM. Finally, most of our patients were male, but this is consistent with the epidemiology of COPD in our country.33
Regarding the strengths of our work, it has been carried out with the largest dataset of patients published so far to address the use, effectiveness and safety of inhaled antibiotics in COPD patients. Previous reports had included between 13 and 36 patients.17–20 Furthermore, for the first time we report the therapeutic advantages in patients with an exacerbator phenotype and/or in remission from CBI by PA. Another asset is that the effectiveness did not vary when a sensitivity analysis was performed on those patients who received treatment for at least 3 months. Finally, since inhaled antibiotics are distributed through hospital channels, patient selection bias or loss of information are highly unlikely.
In conclusion, in our cohort of COPD patients, inhaled antibiotics emerge as effective and safe drugs for reducing the number and severity of exacerbations in those patients that present with an exacerbating or infectious profile, especially in those who achieve PA eradication during the treatment, regardless of the presence of bronchiectasis. Extrapolation of these results to the overall COPD patient population will require similar results to be obtained in further studies.
Authors’ ContributionsD.R.C. is the guarantor of the article, data and analysis.
D.R.C., M.A.M.G. and M.M. were involved in the concept and design of the study, as well as the interpretation of the data.
All authors participated in the acquisition of the data, critical revision of major intellectual content, drafting of the manuscript and approval of the version to be published. The work was carried out on behalf of the “Study group on the effectiveness of inhaled antibiotic treatment in COPD”.
Other ContributionsMedical writing services were provided by Neus Cantariño, MSc, from Trialance SCCL (Spain).
Financial DisclosureThis study received unconditional support from CHIESI ESPAÑA, S.A.U., Spain. There was no intervention by the sponsor in the design of the study, collection and analysis of data or elaboration of the manuscript.
Conflicts of InterestD.R.C. has received speaker fees from PARI, Praxis, TEVA and Zambon and research grants from Praxis. M.M. has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols. The remaining authors declare that there is no conflict of interest regarding the content of this article.
Annie Navarro Rolon (Hospital Universitario Mutua de Terrassa, Terrassa); Xuejie Wang (Hospital del MAR-IMIM, Barcelona, Spain); Alicia Marín Tapia (Hospital Universitari Germas Trias i Pujol, Badalona); Myriam Calle Rubio (Hospital Clínico San Carlos, Madrid); María Jesús Linares Asensio (Hospital Fundación Alcorcón, Alcorcón); Iria Pérez Orbis (Hospital Fundación Alcorcón, Alcorcón); Pilar Martínez Olondris (Hospital Plató, Barcelona); Ascensión Hernando Sanz (Hospital 12 de Octubre, Madrid); Alicia de Pablos Gafas (Hospital 12 de Octubre, Madrid); Margarita Marín Royo (Hospital de Castellón, Castellón); Selene Cuenca Peris (Hospital de Castellón, Castellón); Julia Amaranta García Fuertes (Hospital Universitario Araba-Txagorritxu, Vitoria); Casilda Olveira (Hospital Universitario Regional de Málaga, Málaga); Guillermo Bentabol Ramos (Hospital Universitario Regional de Málaga, Málaga); Lirios Sacristán Bou (Hospital Universitario de Ciudad Real, Ciudad Real); Rosa María Girón Moreno (Hospital Universitario La Princesa, Madrid); Sandra Marín Arguedas (Hospital Dos de Maig, Barcelona); Raúl Moreno Zabaleta (Hospital Infanta Sofía, San Sebastián de los Reyes); Sarai Quirós Fernández (Hospital Universitario La Paz, Madrid); Mikel Sarasate (Hospital Universitari Vall d’Hebron, Barcelona); María Victoria Leal Arranz, MD, PhD (Pneumology Service, Hospital Universitario de Basurto, Bilbao); Gema Castaño de las Pozas (Hospital de Jarrio, Asturias); Nuria Bruguera Ávila (Hospital Sant Jaume de Calella, Barcelona); Carlos Antonio Amado Diago (Hospital Universitario Marqués de Valdecilla, Santander); Soledad Alonso Viteri (Hospital Universitario de Torrejón, Torrejón de Ardoz); María Isabel Ramos Cancelo (Hospital Clínico, Valladolid); Carolina Gotera Rivera (Hospital Universitario Fundación Jiménez Díaz, Madrid); Javier de Miguel Díez (Hospital Gregorio Marañón, Madrid); Gemma Sánchez Muñoz (Hospital Gregorio Marañón, Madrid); Esperanza Martín Zapatero (Hospital Sant Joan de Déu, Manresa); Sandra Ros Celis (Hospital Sant Joan de Déu, Manresa); Silvia Merlos Navarro (Hospital Universitario Virgen de las Nieves, Granada), Rut Ayerbe García (Hospital Universitario Virgen de la Macarena, Sevilla, Spain).
A list of Members of the “Study group on the effectiveness of inhaled antibiotic treatment in COPD” is provided in Appendix A.