Oxygen therapy is defined as the therapeutic use of oxygen and consists of administering oxygen at higher concentrations than those found in room air, with the aim of treating or preventing hypoxia. This therapeutic intervention has been shown to increase survival in patients with chronic obstructive pulmonary disease (COPD) and respiratory failure. Although this concept has been extended by analogy to chronic respiratory failure caused by respiratory and non-respiratory diseases, continuous oxygen therapy has not been shown to be effective in other disorders. Oxygen therapy has not been shown to improve survival in patients with COPD and moderate hypoxaemia, nor is there consensus regarding its use during nocturnal desaturations in COPD or desaturations caused by effort. The choice of the oxygen source must be made on the basis of criteria such as technical issues, patient comfort and adaptability and cost. Flow must be adjusted to achieve appropriate transcutaneous oxyhaemoglobin saturation correction.
Se define como oxigenoterapia el uso terapéutico del oxígeno y consiste en su administración a concentraciones mayores de las que se encuentran en el aire ambiente, con la intención de tratar o prevenir las manifestaciones de la hipoxia. Esta medida terapéutica ha demostrado aumentar la supervivencia en los enfermos con enfermedad pulmonar obstructiva crónica (EPOC) e insuficiencia respiratoria. A pesar de que este concepto se ha extendido por analogía a la insuficiencia respiratoria crónica originada por otras enfermedades respiratorias y no respiratorias, la efectividad de la oxigenoterapia continua no está demostrada en otras entidades. La oxigenoterapia no se ha demostrado efectiva en términos de supervivencia en pacientes con EPOC e hipoxemia moderada. Tampoco hay consenso sobre su empleo durante las desaturaciones nocturnas en EPOC y durante las desaturaciones al esfuerzo. La elección de la fuente de oxígeno se debe realizar por criterios técnicos, de comodidad y adaptabilidad del paciente y de coste. Se debería ajustar el flujo para conseguir una adecuada corrección de la saturación transcutánea de oxihemoglobina.
Oxygen therapy is an ancient treatment, but it still remains one of the most important measures in the management of patients with progressing chronic respiratory disease. The basic goal of oxygen therapy is to correct the severe hypoxaemia that these patients often present in advanced stages of the disease, ultimately improving tissue oxygenation.
The discovery of oxygen is attributed to Joseph Priestley in 1772 who, by heating mercuric oxide in a vessel left in the sun, released gas that proved to be oxygen. However, the first therapeutic use of oxygen is attributed to Chaussier who, in 1780, applied it to dyspnoeic patients and cyanotic new-borns. In 1887, Dr. Holzapple used oxygen generated from potassium chlorate and manganese dioxide to treat a young man suffering from pneumonia. In the late nineteenth century, the process for producing liquid air by compression and cooling was discovered, so oxygen could be isolated by fractional distillation of liquid air. Throughout the twentieth century, the beneficial effects on some of the most common consequences of the disease (less polycythaemia, control of cor pulmonale episodes, and fewer episodes and days of hospitalisation) were demonstrated. However, it was not before the eighties that several studies established the criteria for the selection of patients who would benefit from the use of home-based oxygen therapy (HOT) that are still in use.1
As we describe in these guidelines, there are some clearly defined indications for oxygen therapy, but there are other situations in which there is no consensus on the use of this technique. It is noteworthy that some of these indications are based on studies conducted more than 30 years ago, in some cases, in a limited number of patients. These guidelines aim to serve as a simple and useful tool to assist in decision making when prescribing this therapy. In order to classify the quality of evidence and strength of available recommendations, the GRADE system has been used for the most relevant issues (Table 1). Furthermore, whenever possible, a recommendation based on the issues discussed and the available evidence has been included at the end of each section.
Classification of Recommendations and Quality of Evidence According to the GRADE System.
| Grade of Recommendation | Level of Evidence | Implications |
| Consistent recommendation,a high quality evidence | Well conducted RCT. Exceptionally well carried out OS | Applicable to most patients in most cases |
| Consistent recommendation,a moderate quality evidence | RCT with limitations or well conducted OS with major defects | Applicable to most patients in most cases |
| Consistent recommendation,a poor quality evidence | At least one important outcome in RCT or OS with major defects | May change when stronger evidence is available |
| Consistent recommendation,a very poor quality evidence | At least one important result in unsystematic clinical observations or very indirect evidence | May change when stronger evidence is available |
| Weak recommendation,b high quality evidence | Well conducted RCT. Exceptionally well carried out OS | May differ depending on circumstances or patients |
| Weak recommendation,b moderate quality evidence | RCT with limitations or well conducted OS with major defects | Other options may be better for some patients under certain circumstances |
| Weak recommendation,c poor quality evidence | At least one important outcome in RCT or OS with major defects | Other options may be equally reasonable |
| Weak recommendation,d very poor quality evidence | At least one important result in unsystematic clinical observations or very indirect evidence | Other options may be equally reasonable |
RCT: randomised controlled trials; OS: observational studies.
Source: GRADE Working Group. Grading of recommendations of assessment development and evaluations. Available at: http://www.gradeworkinggroup.org/.
The main function of the respiratory system is to maintain adequate pulmonary exchange of physiological gases. The arterial oxygenation parameter for assessing lung function is the partial pressure of oxygen in arterial blood (PaO2), because its value is generally determined by the gas-exchange function of the lung. Normal PaO2 values in adults vary slightly with age and are between 100mmHg (13.6kPa, 1kPa=7.5mmHg) and 96mmHg (12.8kPa) at 20 and 70 years of age, respectively. Partial pressure of carbon dioxide in arterial blood (PaCO2) also decreases with advancing age (4mmHg between 20 and 70 years), ranging between 38 and 34mmHg, respectively, with an average of 37±3mmHg (4.9kPa).
Strictly speaking, hypoxaemia refers to a decrease in O2 content and/or PaO2. However, this broad definition is confusing, since the relationship between PaO2 and O2 content is nonlinear and depends on many variables: PaO2 can decrease without significant changes in O2 content, while O2 content can be greatly diminished without changes in PaO2 (anaemia or CO poisoning). To avoid these problems, we will stick to the most widely accepted meaning of hypoxaemia, which is a decrease in PaO2 below normal limits for the subject's age.2 In clinical practice, arterial hypoxaemia exists when PaO2 is lower than 80mmHg (10.7kPa) and arterial hypercapnia when PaCO2 is greater than 45mmHg (6.0kPa), breathing room air at sea level. Decreased PaO2 may be due to multiple factors (Table 2). Respiratory failure is defined as PaO2 values below 60mmHg (8.0kPa). To demonstrate the existence of respiratory failure, arterial blood gas must be determined by transcutaneous puncture, preferably in the peripheral radial artery. Pulse oximetry can be used as an alternative technique, and less than 90% arterial oxyhaemoglobin saturation (SpO2) is accepted as indicative of respiratory failure. SpO2 values are much more variable than PaO2, because they can be influenced by extrapulmonary factors, and they provide no information on PaCO2 nor pH.3
Causes Hypoxaemia and Hypercapnia.
| Mechanism | PaO2 | PaCO2 |
| Decreased inspiratory PaO2 | Reduced | Reduced |
| Alveolar hypoventilation | Reduced | Greatly increased |
| Diffusion limitation | Reduced | Without changes or reduced |
| Imbalance VA/Q | Reduced | Increased or reduced |
| Short circuit | Reduced | Reduced |
PaCO2: arterial carbon dioxide pressure; PaO2: arterial oxygen pressure; VA/Q: pulmonary ventilation-perfusion ratio.
From Soler and Rodríguez-Roisin.3
Hypoxia is defined as a decrease in oxygen supply to the cells, which limits the production of energy to levels below cellular requirements. Hypoxia can be produced by various mechanisms (Table 3). The maintenance of the oxygen supply to vital tissues depends on the balance between the harmful effects of hypoxia and the compensatory effects it triggers. The various organs have different degrees of susceptibility to hypoxia, depending on the relationship between metabolic activity, blood flow to the organ, and the possibilities of modifying these factors if necessary. The more rapid the fall in PaO2, the greater the disorders that occur, because acute compensation mechanisms have limited capacity. However, when the onset of hypoxaemia is slow (chronic heart and lung diseases), efficient compensation mechanisms can develop over time.4
Pathophysiological Mechanisms of Hypoxia.
| Hypoxaemic | Decreased FiO2Right-left shuntVA/Q imbalanceAlveolar hypoventilationImpaired diffusionLow CvO2 |
| Anaemic | Decreased haemoglobinCO poisoningMethaemoglobinaemia |
| Ischaemic | Reduced blood flow (shock, heart failure) |
| Tissue toxicity | Cyanide poisoning |
| Excessive tissue consumption of O2 | High fever, energetic muscle exercise |
CO: carbon monoxide; CvO2: oxygen content in venous blood; FiO2: fraction of inspired oxygen; VA/Q: pulmonary ventilation-perfusion ratio.
From Rodríguez-Roisin.2
Decreased PaO2 results in increased ventilation mediated by aortic and carotid chemoreceptors. In the heart, the frequency and strength of contractions rise, thus increasing cardiac output. The heart conduction system is also affected, increasing the frequency of arrhythmias of different types and severity. Pulmonary circulation reacts differently to the other vessels of the body: alveolar hypoxia produces a local increase in pulmonary vascular resistance (which determines the development of pulmonary hypertension), mainly by direct action on the precapillary pulmonary vessels. This constitutes the main mechanism for selective redistribution of pulmonary blood flow to better ventilated areas. In the brain, increased flow is proportionally greater than the increase in cardiac output, due to the decrease in vascular resistance produced by cerebral hypoxia. Along with the myocardium, brain tissue is the most sensitive in the body to lack of oxygen. Another response to hypoxia is the increase in the total volume of red blood cells due to increased production of erythropoietin by the kidney. This increase in oxygen carrying capacity is compensatory, because while each red blood cell carries less oxygen than normal, the increased number of red blood cells reduces the deficit in overall blood oxygen transport.5
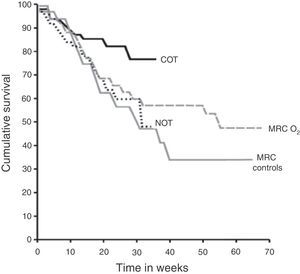
Effects of Oxygen TherapyPatients With Basal HypoxaemiaSurvivalSevere hypoxaemia. The evidence for HOT prescription comes from two randomised clinical trials of patients with COPD published almost 30 years ago: the Medical Research Council (MRC)6 and the Nocturnal Oxygen Therapy (NOTT)7 studies (Fig. 1). The MRC study compared the use of oxygen for 15h per day (including night) with standard treatment without oxygen. This study included 87 patients with severe COPD under 70 years of age with severe hypoxaemia (PaO2 49–52mmHg, 6.5–6.9kPa), hypercapnia (PaCO2 56–59mmHg, 7.5–7.7kPa) and mild pulmonary hypertension. Three-year survival of the group receiving oxygen therapy was 55%, versus 33% in the control group (P<.05). The other study, NOTT,7 in 203 subjects, aimed to evaluate whether continuous oxygen therapy was superior to nocturnal oxygen. The group participants receiving continuous oxygen therapy ended up receiving oxygen 17.7±4.8h per day, while patients on NOTT received 12.0±2.5h per day. During follow-up (mean 19.3 months), survival in the continuous oxygen therapy group was better than in the nocturnal oxygen group (Fig. 1). Overall, the results of these two trials suggest that, in patients with COPD and severe hypoxaemia at rest, oxygen therapy provides a clear survival benefit when administered at least 15h a day, including at night.
Long-term survival with oxygen in males with COPD and severe hypoxaemia. Medical Research Council (MRC) and the Nocturnal Oxygen Therapy (NOTT) controlled long-term oxygen therapy studies. COT: continuous oxygen therapy group in the NOTT study; MRC controls: group receiving no oxygen in the MRC study, MRC O2: group with oxygen therapy 15h a day, including at night, of the MRC study; NOT: nocturnal oxygen therapy group in the NOTT study. From the Report of the Medical Research Council Working Party6 and Nocturnal Oxygen Therapy Trial group.7
Unlike patients with COPD, there is no evidence that oxygen therapy influences the long-term survival or health-related quality of life of patients with interstitial disease and moderate to severe hypoxaemia.8 In a study in patients with idiopathic pulmonary fibrosis (IPF), no differences were observed in health-related quality of life between patients who received oxygen and those who did not.9
Moderate hypoxaemia. In contrast with the above-mentioned results, oxygen therapy has not been shown effective in terms of survival in patients with COPD and moderate hypoxaemia.7,10–12 In a study of 135 patients with PaO2 levels between 56 and 65mmHg (7.5–8.7kPa), patients were randomised to receive oxygen 17h per day or no supplemental oxygen. Survival was similar between both groups during the observation period (mean 41 months).11 Another study aimed at determining the effects of NOTT in COPD patients with mild to moderate daytime hypoxaemia (PaO2 56–69mmHg [7.4–9.2kPa]) and nocturnal desaturation, in whom sleep apnoea–hypopnea syndrome (SAHS) had been ruled out, found no differences in survival or pulmonary haemodynamics.10 In another small study12 of 28 patients with severe COPD and moderate hypoxaemia (PaO2, 66±6mmHg [8.5±0.8kPa]), patients were randomised to receive oxygen 15h a day or no supplemental oxygen. Mortality was similar in both groups after a follow-up of three years.
Pulmonary HaemodynamicsA classic study13 evaluated 16 patients with severe COPD; all had undergone right heart catheterisation (T1) 47±28 months before starting oxygen therapy. At that time their PaO2 was 59±9mmHg and their mean pulmonary arterial pressure (mPAP) was 23±7mmHg. At the start of treatment (T2), catheterisation was repeated. PaO2 was then 50±7mmHg and mPAP 28±7 (P<.008). They received oxygen 15–18h a day, and after 31±19 months (T3), catheterisation was repeated. From T2 to T3, PaO2 remained stable, whereas mPAP was reduced to 25±7mmHg (P<.05). The longer study14 included haemodynamic data from patients with COPD and hypoxaemia (PaO2 55±6mmHg) receiving oxygen (14.7h per day on average). mPAP was measured every two years for six years. In the 39 participants in whom right catheterisation was performed after two years, mPAP decreased slightly from 25±8 to 23±6mmHg. In the 12 patients who completed six years, mPAP decreased from 25±7 to 21±4mmHg at two years, but thereafter returned to baseline values (mPAP 26±7 and 26±6mmHg at 4 and 6 years of follow-up). The authors concluded that oxygen administration in patients with COPD decreased short-term mPAP, followed by stabilisation despite the progression of obstruction.
Patients With Hypoxaemia During Physical ActivitySurvivalSeveral studies suggest that desaturation during exercise is an indicator of poor prognosis in patients with COPD or IPF.10,15–24 However, there is no evidence that oxygen therapy has an effect on survival in these patients. A retrospective substudy of 471 patients with hypoxaemia during arm exercise on medical treatment in the National Emphysema Treatment Trial (NETT) found no differences in survival between subjects who had been treated with or without oxygen. Therefore, although exercise-induced hypoxaemia in patients with COPD or IPF who remain normoxaemic at rest suggests poor prognosis, no prospective data on the effect of oxygen therapy on survival in these patients are available.
Exercise CapacitySupplemental oxygen improves exercise capacity in the short-term management of patients with COPD, cystic fibrosis and interstitial diseases that have only exercise-induced hypoxaemia.25–30 The mechanism appears to be due to its effects on breathing patterns, as suggested by a study in which supplemental oxygen increased tolerance to resistance exercise, and reduced both respiratory rate and dynamic hyperinflation during exercise in patients with COPD and mild hypoxaemia.31 Interestingly, cerebral saturation decreases during exercise in patients with exercise-related desaturation.32 Oxygen supplementation improves cerebral oxygenation and, therefore, it might help maintain brain function during stress.
Few studies have examined the long-term effect of oxygen therapy on exercise capacity in patients who desaturate during exercise. A 12-week, double-blind, randomised, crossover study compared oxygen therapy with air in 26 patients with almost normal SpO2 at rest (94±2.1%) and desaturation during exercise. Oxygen was found to have an acute effect on the distance walked in 6min, but had no long-term benefit in exercise capacity, dyspnoea and quality of life. In another small study of patients with severe COPD and moderate hypoxaemia (PaO2 66±6mmHg [8.5±0.8kPa]), 28 patients were randomised to receive oxygen 15h a day or not for three years. After one year, the cycle ergometer exercise endurance and exertional dyspnoea were better in patients who received oxygen.10 Therefore, little information is available on the benefits of long-term administration of oxygen in exercise capacity and what there is, is contradictory.
Studies on the effect of oxygen therapy on the effectiveness of pulmonary rehabilitation in patients with hypoxia during exercise and without baseline hypoxaemia are contradictory, possibly due to methodological variations.33–36 A recent meta-analysis concluded that oxygen seemed to increase the benefits of rehabilitation in patients with exercise-induced hypoxaemia, while recognizing the poor quality and limited nature of the available evidence.37
Health-related Quality of LifeOxygen supplementation for 12 weeks in patients with desaturation during exercise improved their quality of life, as well as their anxiety and depression, according to specific and general questionnaires. However, there was no correlation between the acute effect of oxygen on the distance walked or on dyspnoea in the 6-min walking test (6MWT) and improvement according to those questionnaires. Moreover, 41% of the patients who improved according to the questionnaires refused to continue with oxygen therapy.37,38
Patients With Nocturnal HypoxaemiaSurvivalRetrospective data indicate that survival may be decreased in patients with nocturnal desaturation,39 but only a few studies have examined the impact of nocturnal supplemental oxygen on mortality in patients with COPD and nocturnal desaturation.11,39,40 In patients with mild to moderate daytime hypoxaemia (PaO2 56–69mmHg, 7.5–7.9kPa) and nocturnal desaturation, increased survival with nocturnal oxygen was not observed.10,39
HaemodynamicsSome investigators have reported elevations in pulmonary vascular resistance and mPAP in patients with nocturnal desaturation41 but others have not.42 Similarly, results from studies on pulmonary haemodynamics with nocturnal oxygen are conflicting.10,41 In a study of patients with isolated nocturnal desaturation, the HOT group showed a decrease in mPAP with supplemental oxygen therapy compared with an increase in the group without oxygen.41 By contrast, another study found no change in mPAP of patients with nocturnal desaturation.10
Premature Ventricular ContractionPremature ventricular contractions occur during sleep in 64% of patients with COPD43,44 and are particularly frequent in those with nocturnal hypoxaemia.43 However, one study found that supplemental oxygen did not decrease the average number of ventricular premature beats in the treated group, although 4 of the 10 subjects experienced a 50% decrease in the frequency of premature ventricular contractions.41
Sleep QualitySleep quality is poor in patients with COPD.41,45–47 The results of studies investigating the effects of NOTT on sleep quality are limited and conflicting, with one study showing an improvement in sleep quality45 and another not finding this benefit.48
RecommendationIn patients with COPD and respiratory failure at rest, oxygen therapy produces increased survival when administered at least 15h a day, including at night. There is no evidence that this positive effect on survival occurs in other aetiologies of respiratory failure or in COPD patients with more moderate hypoxaemia.
Indications for continuous HOT in respiratory disease
Chronic Obstructive Pulmonary DiseaseOxygen therapy is the only intervention, in addition to smoking cessation, that reduces mortality in patients with COPD and severe hypoxaemia (consistent recommendation, high quality of evidence). It also attenuates right heart failure caused by cor pulmonale, improves neuropsychological function, and increases exercise tolerance and ability to perform routine activities (consistent recommendation, moderate quality of evidence).
Continuous Oxygen TherapyOxygen administration corrects hypoxaemia only during application, and has no residual effect. When supplemental oxygen is discontinued, hypoxaemia reappears, so for a sustained effect, administration time must be extended for more than 15h a day.
The indication for oxygen therapy in patients with severe hypoxaemia is determined by its effect on survival. As previously discussed, the NOTT7 and MRC6 studies showed that oxygen therapy increases the median survival of patients with COPD when used for a minimum of 15h per day and with a sufficient flow to obtain PaO2 of 60mmHg, which corresponds to SpO2 ≥92%, without increasing PaCO2. These two studies show that HOT improves survival of patients with COPD and PaO2 <55mmHg (7.3kPa).1 The selection criteria of the NOTT study also allowed the inclusion of patients with PaO2 between 55 and 60mmHg with evidence of cor pulmonale, right heart failure or polycythaemia: a beneficial effect of oxygen therapy on survival was also demonstrated in these patients.7
Several studies have evaluated the use of supplemental oxygen in patients with PaO2 between 56 and 65mmHg (7.4–8.7kPa), and the results are uniformly poor.11,12 Górecka et al.11 did not identify increased survival at three years between a group of patients with PaO2 of 56–65mmHg treated with oxygen therapy and a control group. Similarly, Haidl et al.12 did not find better survival at three years in a small group of patients with COPD and mild to moderate hypoxaemia treated with oxygen compared to a control group, despite the detection of a slight improvement in tolerance exercise and dyspnoea. Therefore, according to the information available to date, continuous oxygen therapy does not improve survival in patients with COPD and mild to moderate hypoxaemia.1 However, the above studies have several limitations. They lack sufficient power to rule out a beneficial effect and, moreover, a longer use of oxygen may possibly be required for achieving beneficial effects (in all of these studies the average was less than 15h per day). The existence of subgroups of patients responding to oxygen therapy cannot be ruled out in these cohorts and patients with COPD and comorbidities, especially cardiovascular and cerebrovascular diseases, which may be more sensitive to the effect of this intervention, are not evaluated.
In the absence of a demonstrated effect on survival in this group of patients, more information on the effect of oxygen therapy on other clinical variables is needed.49,50 Despite the great heterogeneity of the clinical trials analysed, a recent meta-analysis showed that oxygen therapy produces a significant improvement in dyspnoea.51 Moreover, very partial data suggest that it may improve health-related quality of life, increased exercise tolerance, decreased frequency of hospitalisations, and improve depression and cognitive function of these patients.49,52 Its hypothetical role in stabilising pulmonary hypertension and in reducing arrhythmias and electrocardiographic changes suggestive of myocardial ischaemia has not yet been convincingly established.13,14 The LOTT study (NCT00692198) may provide relevant information on these issues in the coming years.
Accordingly, continuous oxygen therapy is indicated in patients with COPD who, at rest and breathing room air, maintain PaO2 less than or equal to 55mmHg (consistent recommendation, high quality of evidence), and in those patients who have PaO2 between 55 and 59mmHg, but also show clinical or electrocardiographic evidence of pulmonary hypertension, haematocrit higher than 55% or signs of right heart failure (consistent recommendation, moderate quality of evidence). Continuous oxygen therapy in patients with COPD and moderate hypoxaemia is not recommended (consistent recommendation, low quality of evidence) (Table 4).53–56
Indications for Oxygen Therapy in Patients with Chronic Obstructive Pulmonary Disease.
| Strength of Recommendation | Quality of Evidence | |
| Continuous oxygen therapy (>15h/day) | ||
| Indicated to improve survival and quality of life when: | ||
| Resting PaO2 ≤55mmHg (7.3kPa), or | Consistent | High |
| Resting PaO2 between 56 and 59mmHg (7.4–7.8kPa) with evidence of organ damage by hypoxia (including right heart failure, pulmonary hypertension or polycythaemia) | Consistent | Moderate |
| Not recommended in patients with COPD and moderate hypoxaemia | Consistent | Poor |
| Oxygen flow should be sufficient to maintain PaO2>60mmHg (8.0kPa) or SpO2>90% | Consistent | High |
| Oxygen therapy during exercise | ||
| May improve the quality of life of patients with exercise desaturation (SpO2≤88%) | Weak | Poor |
| Demonstration of the correction of hypoxaemia during exercise by administering oxygen (SpO2≥ 90%) accompanied by an improvement of dyspnoea or exercise tolerance is required for prescription | Weak | Poor |
| May be useful during exercise in patients in rehabilitation programmes, to increase the duration and intensity of training | Weak | Moderate |
| Nocturnal oxygen therapy | ||
| May be considered in patients with demonstrated nocturnal oxyhaemoglobin desaturation (SpO2< 90% for at least 30% of total recording time) and hypoxia-related sequelae (polycythaemia or signs of right heart failure) | Weak | Poor |
| CPAP or mechanical ventilation should be considered for replacing or supplementing oxygen | Consistent | Moderate |
| Oxygen during air travel | ||
| Requires specifically titrated oxygen flow during sleep, exercise and air travel | Consistent | Poor |
In all cases, the indication for oxygen therapy should be established from a duplicate blood gas quantification within a period of three weeks, during a phase of clinical stability (3months without exacerbation) and on appropriate drug therapy. The oxygen supply should be monitored to ensure that the necessary flow for effectively increasing PaO2 does not trigger acute hypercapnia or acidosis. Finally, the indication for oxygen therapy should be reconsidered in patients who, despite complying with the necessary requirements, continue to smoke, have a clear history of poor compliance or are unable to handle the oxygen supply systems correctly.
Intermittent Oxygen TherapyPatients with baseline PaO2 above 60mmHg may develop severe hypoxaemia in certain circumstances, especially during exercise and sleep, suggesting the need for oxygen administration.
COPD with exercise-induced hypoxaemia. A varying percentage of patients with COPD and mild to moderate hypoxaemia experience desaturation during exercise, which can be identified by a reading of SpO2 ≤88% for at least 2min in the 6MWT.57,58
Reversal of exercise-induced hypoxaemia by oxygen therapy improves peripheral oxygen delivery, reduces ventilatory demand, reduces dynamic hyperinflation and improves right cardiac function.59 Administration of oxygen during exercise in patients who desaturate provides a short-term benefit, with increased exercise tolerance and reduced dyspnoea.60–63 This benefit may be maintained over the medium term, with improvement in health-related quality of life38 and increased exercise capacity.58 However, although exercise desaturation in patients with COPD who remain normoxaemic at rest is a poor prognostic factor, oxygen therapy has not been shown to alter their survival.58
In the absence of more specific information, oxygen therapy during exercise may be considered for increasing the quality of life of patients with exercise-induced hypoxaemia if hypoxaemia correction (SpO2 ≥90%) and improvement in dyspnoea or exercise tolerance by oxygen administration (increase of the distance in at least 25–30m64,65) can be demonstrated (weak recommendation, low quality of evidence).
In patients with exercise limitations due to hypoxaemia, oxygen administration during exercise may allow an increase in effort intensity, and reduce dyspnoea; this should facilitate rehabilitation, the effectiveness of which is amply demonstrated.55,56 The effect of training with supplemental oxygen versus air in patients without severe hypoxaemia has been evaluated in several short-term studies. It has been shown that oxygen increases peak power and endurance time in a constant load exercise, while decreasing isotime respiratory rate,36 and also dyspnoea.62 A meta-analysis of the effect of oxygen therapy during a training programme confirms that it improves endurance time and reduces dyspnoea during constant load exercise, and during the shuttle walking test, whereas no effect was identified on the progressive cardiopulmonary exercise test or on 6MWT.37 However, there are no studies showing that patients performing rehabilitation without oxygen have worse outcomes than patients receiving oxygen. Therefore, administration of oxygen during exercise in patients with exercise desaturation included in rehabilitation programmes is recommended in order to increase the duration and intensity of training (weak recommendation, moderate quality of evidence) (Table 4).
COPD with nocturnal hypoxaemia. There are several criteria for the identification of nocturnal hypoxaemia, including an episode of desaturation of at least 5min duration with minimum SpO2 ≤85% observed at least once during the night, preferably during REM sleep.39 Although the most operational definition consists of SpO2 <90% for a duration ≥30% of the total recording time,66 patients with COPD and moderate hypoxaemia during wakefulness have been shown to have worse survival at three years if they have nocturnal desaturation than those without it.39
There is still little information available on the effect of NOTT. Two small studies have detected no effect on survival or on the indication of continuous oxygen therapy.10,39 Although their sample sizes are inadequate for assessing the incidence of these episodes, currently available evidence suggests that NOTT does not improve survival of patients with COPD who have only nocturnal desaturation. Results from the evaluation of other possible effects of NOTT are controversial and provide no consistent information about its impact on the quality of sleep and the development of arrhythmias or pulmonary hypertension.58 While some authors describe a minimum effect on increased pulmonary pressure,40 another study did not detect an impact on pulmonary haemodynamics.10 In coming years, the INOX study (NCT01044628) is expected to provide more solid information for determining whether NOTT affects mortality or the future need for oxygen therapy in patients with COPD associated with moderate hypoxaemia and nocturnal desaturation.
NOTT can be considered in patients with demonstrated nocturnal oxyhaemoglobin desaturation (SpO2 <90% for at least 30% of total recording time) and hypoxia-related sequelae (polycythaemia or signs of right heart failure) (weak recommendation, low quality evidence). In this situation, CPAP or mechanical ventilation should be considered for replacing or supplementing oxygen therapy (consistent recommendation, moderate quality of evidence) (Table 4).
Other special situations. Patients with severe COPD and moderate hypoxaemia at sea level may require oxygen supplementation during air travel, especially during overseas flights. Assessment of the oxygen needs and the flow titre needed during air travel should be made according to specific recommendations.67,68
Other Respiratory ConditionsThe concept of increased survival in COPD patients treated with oxygen therapy has been extended by analogy to chronic respiratory failure caused by other conditions.69 While this concept seems reasonable, it should be remembered that it is based on the hypothesis that the beneficial effect of oxygen is due to hypoxaemia correction, regardless of its cause, but this has not been demonstrated. Preliminary information is available on the role of oxygen therapy in some diseases.
Pulmonary HypertensionThere are no consistent data on the long-term effects of oxygen therapy in patients with pulmonary hypertension.70 Although an improvement in pulmonary hypertension with low oxygen flow has been described in some patients with pulmonary arterial hypertension,13,71 this has not been confirmed in controlled studies. In a controlled study in patients with Eisenmenger syndrome, NOTT showed no effect on haematological variables, quality of life or survival,72 while a previous study suggested increased survival.73
In these patients, oxygen therapy is indicated if PaO2 is less than 60mmHg, and the aim is to maintain SpO2 >90% (consistent recommendation, low quality of evidence).74–76 Oxygen therapy during exercise can be considered when symptomatic benefit of the exercise desaturation correction is demonstrated (weak recommendation, low quality of evidence).76
Diffuse Interstitial Lung DiseaseHypoxaemia at rest and desaturation during exercise in patients with IPF are factors of poor prognosis,77,78 so reversal may be of clinical interest. Moreover, oxygen therapy may attenuate the component of pulmonary hypertension caused by hypoxaemia and help improve exercise tolerance and quality of life of these patients.79 However, oxygen administration has not been shown to improve the survival of IPF patients.80,81
In the absence of specific data, HOT is recommended in case of severe hypoxaemia at rest (PaO2 <60mmHg) or desaturation during exercise (consistent recommendation, quality of evidence very low).82
Cystic FibrosisNOTT has shown no effect on mortality, hospitalisations and disease progression in patients with cystic fibrosis, although it reduces school and workplace absenteeism.83 In turn, oxygen administration during exercise seems to improve respiratory and cardiovascular work, increased exercise capacity, and reduced oxyhaemoglobin desaturation.27,84 The wide heterogeneity of the studies is evident in a meta-analysis confirming that oxygen therapy shows no effect on survival but reduces absenteeism.85 In addition, it shows that oxygen therapy during exercise can increase its duration.85
Therefore, in the absence of more specific information, oxygen therapy is recommended for severe hypoxaemia (PaO2 <60mmHg), with special emphasis on the need for correct titration of the oxygen flow required during exercise (consistent recommendation, quality low evidence).
Reassessing the IndicationOnce oxygen therapy is indicated, re-evaluation after a month or two months from prescription is recommended in order to verify compliance, ensure that the patient continues to refrain from smoking, and to evaluate the clinical impact, both in perception of subjective benefit, and the effect on patients’ quality of life. Baseline blood gas assessment is required to rule out sham severe hypoxaemia, which can cause up to 30% of unnecessary prescriptions during hospital discharge.86,87 In addition, blood gas assessment with the prescribed oxygen flow is recommended to verify that PaO2 is maintained at >60mmHg.
Although there is no consensus recommendation on the frequency of periodic reviews, at least one annual visit seems reasonable.
RecommendationContinuous oxygen therapy is indicated in patients with COPD with PaO2 ≤55mmHg at rest or PaO2 at rest 56–59mmHg, with evidence of organ damage by hypoxia. Desaturation during exercise can improve quality of life in patients who experience desaturation during exercise (SpO2 ≤88%). For prescription, demonstration of improvement in dyspnoea or exercise tolerance with oxygen administration is required. NOTT may be considered in patients with nocturnal oxyhaemoglobin desaturation (SpO2 <90% for at least 30% of total recording time) and hypoxia-related sequelae.
Indications in Other SituationsThe recommendations and available evidence for the use of oxygen in other clinical situations are discussed below.
Congestive Heart FailureData from randomised controlled studies on the role of HOT in patients with congestive heart failure show no benefits on survival or functional status of patients (consistent recommendation, very low quality of evidence).
Up to 33–82% of patients with chronic heart failure have been reported to possibly have central apnoea with Cheyne-Stokes periodic breathing.88,89 Suppression of nocturnal hypoxaemia with oxygen therapy improves Cheyne-Stokes respiration,89–93 reducing sympathetic activity and increasing exercise tolerance.89 In these patients, nocturnal oxygen has been shown to improve sleep parameters, left ventricular function and health-related quality of life.89,94,95
Therefore, in patients with heart failure (left ventricular ejection fraction <45%) and Cheyne–Stokes respiration, NOTT should be considered, once correction of sleep parameters has been verified. It should be used preferably in combination with forced ventilation (consistent recommendation, high quality of evidence).
Hepatopulmonary SyndromeThe hepatopulmonary syndrome (HPS) is characterised by an increased alveolar–arterial oxygen gradient caused by pulmonary vasodilation. Initially, it may present without hypoxaemia and occurs in the context of liver disease, both acute and chronic, particularly liver cirrhosis. The cause of hypoxaemia in HPS is the dilation of precapillary and postcapillary lung vessels that allow the quick passage of desaturated venous blood to the pulmonary veins, resulting in decreased oxygenation of arterial blood. Most studies have found that cirrhotic patients with HPS have higher mortality than patients without HPS with a similar degree of liver dysfunction. The prognosis is worse in those with a PaO2 <60mmHg.96
HPS intensity is established whenever an important alveolar–arterial O2 gradient is found (>15mmHg), depending on the degree of hypoxaemia: mild when PaO2 >80mmHg, moderate between 60 and 80mmHg; severe from <60 and more than 50mmHg, and very severe when less than 50mmHg. The treatment of choice is liver transplantation.96
Administration of O2 has been shown to provide symptomatic relief in isolated cases, although no evidence for widespread use is available. Improved liver function has been described in two patients treated with oxygen, reinforcing the concept that hypoxia can directly alter liver function and regeneration. The recommendations of scientific societies establish that HOT should be indicated in patients with PaO2 between 50 and 60mmHg, whereas the indication must be individualised in those with severe hypoxaemia (consistent recommendation, very low quality of evidence).97
Cluster HeadacheThis entity is listed as one of the most excruciating pains that a human being can experience. Pharmacological treatment of choice during crises is the triptans (sumatriptan). This medication is subcutaneously injected and takes effect in only 15min. However, it is contraindicated in patients with ischaemic heart disease, and its use should be confined to two injections per day. The alternative is inhalation of 100% O2.98 The great advantages of O2 is that it can be combined with drug therapy, it can be used several times a day, it has no side effects, and can be used in patients in whom triptans are contraindicated.99 Controlled studies have shown that administration of 100% O2 for 15min at the beginning of the episode is a safe and effective treatment in terms of aborting the crisis, and this has been reflected in practice guidelines.100 Results of a double-blind, placebo-controlled, crossover study providing strong scientific evidence on the superiority of the O2 administration versus air in the elimination of pain at 15min and a significant improvement in pain in this period have been recently published.101 The door remains open to studies for establishing the best way to administer O2 and the domiciliary dose for patients with cluster headaches (consistent recommendation, moderate quality of evidence).
Dyspnoea Secondary to CancerIt is assumed that oxygen administration to cancer patients with dyspnoea may partially alleviate their symptomatology.102 Consequently, oxygen therapy is considered a palliative treatment in various guidelines.103,104
However, two meta-analyses failed to verify the effectiveness of oxygen as symptomatic treatment of refractory dyspnoea in cancer patients without severe hypoxaemia.105,106 Neither did Abernethy et al.107 demonstrate the superiority of oxygen versus air in the control of symptoms in cancer patients with limited life expectancy, refractory dyspnoea and PaO2 >55mmHg. A recent meta-analysis evaluating the efficacy of various treatments for symptomatic dyspnoea secondary to cancer confirms the beneficial effect of opioids, while no benefit is detected for oxygen.108
Therefore, oxygen is less effective than opiates for the symptomatic treatment of secondary dyspnoea in cancer (consistent recommendation, high quality of evidence), and it may be considered only if an additional effect is identified in a short therapeutic trial (weak recommendation, low quality of evidence).
Oxygen Therapy in ChildrenOxygen treatment is somewhat different in children compared to adults.109 Most clinical situations that require the administration of oxygen to children are unique to paediatric patients, although there is occasionally some overlap between older children and young adults. Prognosis is usually good and many children need oxygen only for a limited period, determined by growth and neurodevelopment. Furthermore, many children only require oxygen at night, usually for less than 15h.110,111
Specific indications for oxygen therapy in children are limited to three conditions: severe hypoxaemia (3 standard deviations below expected values with the child in a stable condition on room air); nocturnal desaturation (total time with SpO2 <90% for longer than 20% of the recording time); or presence of pulmonary hypertension, right ventricular hypertrophy or polycythaemia secondary to chronic hypoxaemia (consistent recommendation, moderate quality of evidence).110,111
Oxygen therapy reduces or prevents pulmonary hypertension in neonatal chronic lung disease. It reduces intermittent desaturations, decreases airway resistance and promotes growth (consistent recommendation, low quality of evidence). It is also beneficial for neurodevelopment (consistent recommendation, very low quality of evidence), may reduce the associated risk of sudden death (consistent recommendation, very low quality of evidence) and decreases hospitalisation (consistent recommendation, low quality of evidence).110,111 In children with cystic fibrosis, oxygen therapy improves school attendance (consistent recommendation, moderate quality of evidence), and produces symptomatic improvement (consistent recommendation, very low quality of evidence).111
RecommendationNOTT should be recommended for patients with congestive heart failure and diagnosed Cheyne-Stokes respiration, and preferably associated with forced ventilation. Indication of HOT in patients with HPS should be considered in those with respiratory failure. Administration of 100% oxygen has proved efficacy in patients with cluster headaches. Palliative use of oxygen therapy in cancer patients with dyspnoea has proved less effective than opioid administration.
Sources of OxygenStatic Systems (Fig. 2)Gas CylindersAluminium cylinders have replaced steel cylinders for storing compressed gas at home (200 bars). Several sizes are available, which is useful for patients with low mobility. HOT is currently administered using preferably two types of static systems: oxygen concentrators or liquid oxygen reservoirs (Fig. 2).
Oxygen ConcentratorsThese devices are connected to the main power supply. They weigh 13–26kg and use the air separation technology to separate nitrogen from ambient air. Concentrators are able to deliver 95% pure oxygen at a rate of 3–4l/min, although some models provide up to 10l/min. They are useful in patients who require low flows and leave home sporadically. They cost less than cylinders, because they require fewer deliveries from the supplier. To facilitate movement in the family environment, patients can connect their stationary system with a tube of up to 15m in length. This applies to any static supply system.112
Liquid OxygenLiquid oxygen can be stored, transported and transferred to other devices more efficiently than gas systems. With an expansion ratio of 860:1, 1l of liquid oxygen will expand to 860l of oxygen gas, and can provide a continuous flow of up to 15l/min of 99% oxygen. The main component is a base unit (called “mother unit”), which is a specially designed container that stores liquid oxygen at −180°C and is accompanied by a backpack for ambulation. This method is recommended for patients requiring oxygen outside their home and high flows at rest (>3l/min). However, as the oxygen content depletes, the mother unit requires periodic refilling by the home oxygen suppliers (Table 5).
Main Characteristics of the Oxygen Sources.
| Compressed Gas Cylinder | Portable Gas Cylinder | Concentrator | Liquid Oxygen | |
| Indications | Patient without mobility | Complements fixed source to ensure mobility | Patient with low mobility and low flows | Patient with good mobility |
| Advantages | Noise-free | Mobility outside the home | No need for distribution network | Mobility outside the home. Acceptable autonomy. Refillable from mother unit. |
| Disadvantages | Distribution network. Static source | Weight. Distribution network. Limited autonomy. Not refillable. | Loss of efficiency with high flows. Noise. No mobility outside the home. Main electricity | Distribution network |
| Cost | Medium | Medium | Low | High |
HOT should not be interrupted when patients need to leave their home for a few hours. Therefore, patients require portable oxygen delivery systems as part of their prescription.113,114
A classic method is the use of an oxygen gas cylinder the size of a small backpack (size M-6) that, if necessary, can be transported in a trolley. However, its small capacity comprises a limitation, as a patient with a HOT prescription of 2l/min depletes an M-6 (164l of oxygen gas) cylinder in just over an hour. Larger cylinders are extremely unwieldy and difficult to transport.
The second option is a portable backpack that can be attached to the aforementioned liquid oxygen mother unit. This can be filled and used by active patients with demonstrated reduction in oxyhaemoglobin saturation (SpO2) during exercise who want to use it away from home.115–117 Usage time will depend on the flow used. It is useful for short trips by patients who need high flows of more than 3l/min during exercise.
Concentrator with cylinder transfer. This is an adaptation of the traditional concentrator. This module transfers O2 to a portable cylinder, and requires several hours for filling and daily refilling. It is useful for patients who sporadically leave home, although this system is rare in our country.
Portable oxygen concentrator. This has the advantage of being adaptable to any power outlet or car battery. This device has led to the technology called “non-delivery/delivery-less”, because it does not need gas to be delivered to the home, but it does require servicing every three months. Portable concentrators should weigh a maximum of 4kg, although some continuous-flow systems weigh 9kg and produce 90±3% oxygen, providing at least 2 litres of oxygen for at least 4h. They are useful in patients who make long journeys and frequently participate in activities away from the home.117
Oxygen Conservation Technology (Fig. 3)On-demand valve. The so-called “pulse dose systems”, “demand flow”, “demand oxygen delivery system” and/or “oxygen-conserving devices”, unlike continuous flow therapy, supply oxygen intermittently. Instead of a continuous flow, the on-demand oxygen storage technology (OST) provides a pre-set volume or oxygen bolus, measured in millilitres per breath. The bolus is delivered when the patient makes an inspiratory effort (or demand), during the first 60% of inspiration. Hence, oxygen is not wasted for the remainder of each breathing cycle, allowing a longer duration of the supply source. Importantly, the numerical setting of a continuous flow metre, expressed in litres/min, is not equivalent to the numerically different configuration of an OST, which depends on the model, and indicates the relative volumes of bolus delivered. Two systems can be used: liquid oxygen and concentrator. The first supplies between 1.5 and 5 pulses (pulse of ±15ml), refilling from the corresponding mother unit. Also, some models capable of functioning both with pulse or continuous flow are available. Pulses vary depending on models in the case of portable oxygen concentrators (1–6 pulses), with different pulses. These have the advantage of being easily refilled and used at home and on travel. Some models can be used for both continuous flow and demand. They are useful in patients capable of triggering the valve in whom correct saturations during ambulation have been verified118 (Fig. 3).
On-demand devices are sensitive to variables such as the patient's respiratory rate, which may affect the SpO2 figures obtained. Therefore, alarms should be set to trigger when SpO2 is lower than 85%. For the prescription of all portable systems, evaluation of the patient during exercise for proper dose adjustment is required.119
Systems for Delivery to the PatientTranstracheal CatheterThe transtracheal catheter delivers oxygen directly into the trachea through a small catheter 1.6–2mm in diameter that is introduced percutaneously between the second and third tracheal rings. This results in oxygen saving of 50% at rest and 30% during exercise. It is indicated for patients using portable sources for ambulation. The drawbacks are that it is an invasive method, requiring training and education for care, and it must be replaced every 60–90 days in a hospital setting.50 Current use is limited.120
Venturi MaskThe fraction of inspired O2 (FiO2) is regulated by opening the side windows of the “Venturi effect” mask. It provides a fixed, constant supply of FiO2, irrespective of the patient's breathing pattern. As home treatment, this is only used in patients with hypercapnia, and applied overnight.
Nasal ProngsThis is the commonly used system at home. It consists of two small, flexible tubes 0.5–1cm in diameter that are inserted in the nostrils and adjusted behind the ears. FiO2 is completely variable, depending on the patient's tidal volume. Since it is recognised that standard nasal prongs are inefficient (only 15–20% of the administered oxygen participates in the gas exchange), oxygen-saving systems have been designed for a more efficient use of oxygen, with an increase in autonomy of up to 3 times longer than continuous oxygen supplied with conventional nasal prongs.
ReservoirsThese use continuous sources, provide an oxygen-enriched bolus at the beginning of inspiration and accumulate an amount of ±20ml during expiration. They are useful for patients who require higher flow rates that cannot be supplied by the usual sources, or those requiring similar SpO2with lower flows121 (Table 6).
Main Features of Oxygen Saving or Release Systems.
| Transtracheal Catheter | Reservoir Cannula | On-demand System | |
| Saving mechanism | Reduces dead space. The upper airway acts as a reservoir | Stores oxygen during exhalation and administers it at the onset of inspiration | Provides oxygen only during inspiration, especially at the beginning |
| Indications | Portable source Refractory hypoxaemia | Portable source Refractory hypoxaemia | Portable source |
| Advantages | Improves lung function parameters | Easy to use | Suitable for liquid oxygen bottles |
| Disadvantages | Invasive | Low comfort | Decreased effectiveness at high respiratory rates |
| Cost | High | Low | Medium |
Washing with soap and water of the part of the delivery systems in contact with the patient (e.g. nasal prongs, reservoir cannulas or masks) every morning is recommended. Extensions and tubes should be washed once a week, and replacement of nasal prongs and face-masks every 15 days is recommended. In the case of reservoir cannulas, the manufacturer recommends replacement every 3 weeks.
With regard to the supply systems, oxygen is not combustible, but activates combustion of flammable materials, so the general recommendations of the suppliers must be adhered to.117
Specific recommendations according to source of oxygen
Gas CylinderAvoid dropping. Never grease or lubricate the supply valves. Open the flow regulator smoothly.
ConcentratorThe concentrator should be placed 15cm from the wall or furniture to facilitate air circulation. It should never be covered. There is a waiting period between 5 and 10min from start-up until it can be used; this time is necessary to accumulate an adequate oxygen concentration. The concentrator should be disconnected when not in use. It can be located in another room or on a mat or rug to reduce the noise. It should be moved vertically, even during transportation. While at home, the portable concentrator should be always connected to the power supply to keep the battery charged. The power cord should be brought along when leaving home in case it is necessary. The air inlet filter should be washed weekly.
Liquid OxygenLiquid oxygen is extremely cold, so the icy parts should not be touched. Backpack filling should be performed in a well-ventilated room with a solid floor. The reservoir should not be left unattended during refilling. If leaks occur while disconnecting the mother unit and the backpack, the pack should be connected again and, if this is not possible, the room should be ventilated. Do not touch the source of the leaks, and do not smoke or cause sparks or flames. In case of leakage, it is recommended to stay away from the outflow. If it comes into contact with the eyes, wash with plenty of water for more than 15min. If liquid oxygen comes into contact with the skin, do not rub the area, remove clothing if necessary, thaw the affected areas with moderate heat and, in both cases, seek medical care.122
Prescribing Continuous Domiciliary Oxygen TherapyAdjustment of Oxygen FlowAlthough oxygen flow should be adjusted to achieve adequate SpO2 correction, there is wide disparity in the methods for achieving this. According to surveys performed by Wijkstra et al.,123 less than 40% of pulmonologists properly adjusted the flow for the different situations of rest, sleep and exercise.
At RestOxygen flow should be adjusted when the patient is not smoking, is in a stable phase, awake, at rest and after pharmacological treatment has been optimised. Appropriate correction of arterial blood gases should also be verified, using the same oxygen supply the patient will use at home.124 Flow adjustment with a pulse oximeter until SpO2 ≥90% is therefore recommended and, at this point, arterial blood gases should be obtained to ensure adequate correction of hypoxaemia without causing hypercapnia (consistent recommendation, high quality of evidence). In patients with normocapnic respiratory failure, adjustment only with the pulse oximeter may be performed.
During SleepAn average SpO2 <90% and/or percentage of time with SpO2 <90% (CT90) >30% is considered as nocturnal desaturation.125
Several methods to adjust oxygen flow during sleep are described:
a) Maintain the same flow as at rest. Various studies126–128 show that daytime-adjusted oxygen flow provides inadequate correction of SpO2 during sleep in a large number (between 16 and 48%) of patients. Ruling out the presence of sleep apnoea-hypopnea syndrome (SAHS) and adjustment during a stable phase of the disease can result in better SpO2 correction.126
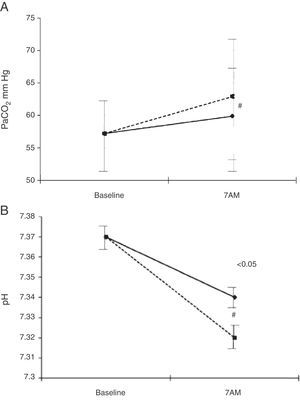
b) Increase oxygen flow at rest in 1 or 2 l/min to prevent a drop in nocturnal SpO2 (consistent recommendation, moderate quality of evidence). This is the recommendation given by some international guidelines129 that claim there is no risk of secondary hypercapnia. However, it is well known that the administration of oxygen can potentially generate hypercapnia. This phenomenon is mediated by different mechanisms such as hypoventilation, alterations in ventilation-perfusion or the Haldane effect. In addition, sleep produces a series of events such as a decrease in basal metabolism, an increase in airway resistance, hypotony of respiratory muscles and decreased sensitivity of the respiratory centre. In COPD patients, an additional issue is greater hypoventilation, due to a reduction in tidal volume, the frequent association of obstructive sleep apnoea and/or reduction in mucociliary clearance. Therefore, NOTT induces hypercapnia in COPD patients more frequently than expected. In fact, two studies127,130 detected nocturnal hypercapnia in between 43 and 59% of patients receiving daytime-adjusted oxygen flow. Predictors of poor response to oxygen are higher BMI and lower PaO2 during daytime oxygen administration131 (Fig. 4).
Evolution of PaO2, PaCO2 and pH in patients with COPD and HOT. Breathing room air; COPD, chronic obstructive pulmonary disease; NHV: nocturnal hypoventilation; HOT: home oxygen therapy; PaCO2: arterial carbon dioxide pressure, PaO2: arterial oxygen pressure. From Tarrega et al.131
It seems obvious that, if oxygen flow is increased to 1 or 2l/min, hypoventilation phenomena might worsen, as demonstrated in a study where a significant increase in PaCO2 and respiratory acidosis was observed in a considerable number of patients after flow was increased by 1 litre compared to daytime settings (34.2% vs. 23.7%).132
c) Adjust oxygen flow according to continuous SpO2monitoring during sleep to maintain SpO2≥90%. The situations described above reinforce the need for the individual assessment of patients with COPD and chronic hypercapnic respiratory failure in order to adjust oxygen flow during sleep. It would be appropriate to establish flow during sleep with a pulse oximeter with the aim of maintaining SpO2 ≥90% and, if the patient has hypercapnia, determination of morning blood gases would be essential to confirm that the prescribed flow does not increase PaCO2 (weak recommendation, low quality evidence).
During ExerciseDesaturation is generally defined as the presence of mean SpO2 ≤88% during a stress test such as the 6MWT, and adjustment of the oxygen flow during this test to average SpO2 ≥90% is recommended124 (consistent recommendation, high quality of evidence). However, this is rarely done.123 Indeed, the recommendations of some European countries suggest using the same oxygen flow indicated at rest or 1l more, without performing any test for assessing the flow required for each patient.
The stress test most commonly used to detect a reduction in SpO2 and to adjust oxygen flow to exercise is the 6MWT, although others, such as submaximal effort on cycle ergometer or treadmill, have been used.133 A good correlation has been shown between SpO2 during 6MWT and that obtained during daily activities, and also in the oxygen flow necessary for correction of such desaturation in both situations.134
Table 7 shows some recommendations for the correct adjustment of oxygen flow at rest, during sleep and during exercise.
Recommended Settings of Oxygen Flow at Rest, During Sleep and During Exercise.
| At rest |
| Clinically stable |
| Receiving optimal pharmacological treatment |
| Smoking cessation |
| Arterial air gases at rest and seated |
| Perform 2–3 determinations of arterial gases breathing air, over a period of a month, demonstrating that conventional COT criteria are met |
| Administer oxygen by nasal prongs and use a pulse oximeter to confirm SpO2 ≥90% |
| Arterial blood gases with adjusted oxygen, confirming a good PaO2 correction without elevated PaCO2 |
| During sleep |
| Continuous monitoring of SpO2 by pulse oximetry |
| Adjust oxygen flow to maintain mean SpO2 ≥90% during sleep |
| Morning arterial blood gases with oxygen flow adjusted by pulse oximetry (in patients with hypercapnia) |
| If PaCO2 increases (≥10mmHg) and/or pH is lowered (poor response): |
| Rule out obstructive sleep apnoea |
| Administer oxygen by Venturi mask or consider NIV |
| During exercise |
| Demonstrate average <88% SpO2 during a stress test (6MWT) |
| Demonstrate SpO2 improvement (>90%) during the stress test (6MWT): performing repeated 6MWT with different oxygen flows to achieve SpO2 ≥90%, with a break of at least 30min between each test |
| Demonstration of increased exercise capacity (metres) |
COT: home-based continuous oxygen therapy; PaCO2: arterial carbon dioxide pressure; PaO2: arterial oxygen pressure; 6MWT: 6min walking test; SAHS: sleep apnoea–hypopnea syndrome; SpO2: oxyhaemoglobin saturation.
From O’Donohue.124
In general, use of oxygen for more than 15h a day is recommended, including night-time administration (consistent recommendation, high quality of evidence). However, careful assessment of NOTT7 and MRC6 results reveals that patients who received treatment for 24h had longer survival, although average use was approximately 18h/day. It stands to reason that the more hours of treatment, the greater benefits can be achieved, especially if it is used during exercise, when the most marked SpO2 reduction occurs. An interesting piece of information extracted from a study by Eaton et al.52 is that the only predictor of response to HOT in terms of health-related quality of life is the number of hours of treatment. This finding supports the fact that hours of treatment have an impact on the benefits.
Currently, thanks to portable devices, oxygen can be used throughout the day, overcoming limitations in daily activities. A major drawback is the difficulty of patient's acceptation of the use oxygen away from home.
Choosing the Oxygen SourceThe choice of oxygen source will depend the patient's profile, his or her capacity and desire for mobility and, above all, the appropriate SpO2 correction at rest and during sleep or exercise.
According to the patient's mobility profile, the following is recommended112:
Patients with no or limited mobility. Static oxygen supply, predominantly concentrator-based, considering that mobility within the home is equipped with an extension tube of up to 15m in length. Provision of a portable oxygen bottle for leaving the home occasionally is advisable. A concentrator with a portable oxygen bottle refill may also be prescribed.
Patients with mobility for short trips only. Portable oxygen, either portable concentrator or liquid, taking into account that the duration of the portable concentrator is 1–3h maximum, depending on the model and the backpack, and that of liquid oxygen is 2–6h, if equipped with a saving valve system and, above all, the flow needed during exercise.
Patients with greater mobility, attending day-care centres or with work activity, more than one home and travel. A portable concentrator is recommended, since it allows connection to mains or car plug. This is the only system permitted for air travel. Both portable concentrator and liquid oxygen can be considered for long boat trips. In both cases, the travel agency should be consulted regarding the acceptability of the oxygen source. Addition of an external battery allows more autonomy but increases the weight of the system.
Two fundamental premises must be added:
The use of a portable concentrator should be restricted to patients requiring low oxygen flow (<3l/min or 6pulses/min, the maximum possible supply, depending on the model), and effectiveness should always be verified with a stress test (achieving SpO2 ≥90%).
In the event that the system is equipped with a demand valve, effectiveness should always be verified with a stress test, both for liquid oxygen and the portable concentrator. This is unsuitable for patients who use CPAP or other mechanical ventilation.
Cost-Efficiency of DevicesDeducing the cost of oxygen therapy is a complex task. It is more complicated than merely assessing the costs indicated in current agreements with various health authorities, whether listed by cost per daily treatment, per capita cost, or alternative cost estimates. The cost of additional technologies and technological innovation must be added to this basic expenditure. Then the benefits of this treatment modality must be subtracted from the costs. To this equation must be added the consideration that the indications are correct, compliance with prescription optimal, and adherence complete. Only then can we be certain that investment in home oxygen therapy is beneficial.
The report from Fenin and PricewaterhouseCoopers in 2011 has provided some data on the efficiency and benefits of home oxygen therapy in Spain.135 In this report, the cost-utility analysis of treating COPD with oxygen shows that the annual treatment of a patient with stage IV COPD (by spirometric obstruction) represents an average saving of 1372euros (€), while improving the patient's quality of life by 0.15 years in quality-adjusted life years per patient. Another important point in this report is that the cost of a patient with stage III COPD represents approximately 70% of the cost of a patient with stage IV COPD. These data indicate that the weighted average cost estimate of a treated stage III-IV patient would be €3178, while the average cost of an untreated patient would be €4079. Overall, 79000 COPD patients are estimated to have received treatment with oxygen in Spain in 2010, representing a cost of 100 million euros, while 42000 patients that should have received this therapy did not, generating an associated cost of 172 million euros.
No data on the cost-effectiveness of specific equipment for oxygen therapy, such as portable oxygen concentrators or liquid oxygen, are available.
RecommendationOxygen flow should be adjusted using the same source the patient will use at home. Adjustment of the flow with a pulse-oximeter until achieving SpO2 ≥90% is recommended, and arterial blood gases should be obtained at this point to ensure adequate hypoxaemia correction without causing hypercapnia. In patients with normocapnic respiratory failure, adjustment can be made using only the pulse-oximeter. Adjustment of oxygen flow during sleep should be performed with continuous SpO2monitoring during sleep, aimed at maintaining SpO2 ≥90%. During exercise, adjustment of oxygen flow for an average SpO2 ≥90% during the 6MWT is recommended. The choice of the oxygen source should be adapted to the patient profile.
Side Effects and Risks of Home Oxygen TherapyLike any drug, oxygen therapy has its side effects and risks,136,137 although it is generally a safe treatment if the basic guidelines are followed. The main adverse effects are derived from equipment misuse.
The side effect that most impacts on the clinical management of patients requiring HOT is hypercapnia. The main mechanisms of this phenomenon are worsening of the V/Q balance secondary to inhibition of hypoxic vasoconstriction and inhibition of hypoxic stimulus. This occurs particularly during sleep127,129 and may worsen if oxygen flow is increased to correct nocturnal hypoxaemia as recommended by various guidelines, as recently demonstrated in COPD patients in stable phase and chronic hypercapnic respiratory failure132 (Fig. 5).
Evolution of PaCO2 (A) and pH (B) during sleep with flow adjusted during waking (solid line) and after increasing it by 1l/min during sleep (dotted line). From Samolski et al.132
The clinical impact of nocturnal hypercapnia secondary to oxygen therapy is not known. However, presence of daytime hypercapnia has been considered a factor for poor prognosis in the outcome of COPD patients who require HOT.138–140 Hypercapnia may enhance muscle dysfunction, reduce diaphragm contractility and lead to muscle fatigue.141,142 It may also influence cardiac contractility, leading to arrhythmias and structural injuries in the myocardium.143–145 Other known effects of hypercapnia are decreased cerebral vascular resistance with increased intracranial pressure, enhancing cerebral tissue hypoxia.
In the case of concurrent SAHS and COPD, oxygen therapy may prolong the duration of obstructive events, even with low flows.146,147 As a result of these phenomena, the sleep quality of these patients could be affected.148
Another side effect of oxygen therapy is pulmonary toxicity. Oxygen can cause direct lung injury by causing absorption atelectasis, since the intra-alveolar nitrogen concentration is reduced, and diffuse lung injury (acute and chronic) caused by release of free radicals. These complications are typical of prolonged oxygen administration at high concentrations. Such alterations are dose-dependent, and related both to partial pressure of oxygen and exposure time. Lesions are irreversible only in cases of chronic lung injury (capillary proliferation, interstitial fibrosis, epithelial hyperplasia and bleeding). Although HOT uses low O2 flows and exposures to FiO2 <0.5 that can be tolerated for weeks, cases of patients receiving HOT who have presented histological changes related to oxygen toxicity have been described.149
Finally, other adverse effects that may influence treatment compliance are congestion and irritation of nasal mucosa and epistaxis, contact eczema from the nasal prong material, and psychological and social effects.
There is also a risk of fire and explosions that is much more acute when the patient continues to smoke. Liquid oxygen can cause burns when handling the supply source or when there are leaks in the system.
Conflicts of InterestThe authors declare no conflicts of interest.
Please cite this article as: Ortega Ruiz F, Díaz Lobato S, Galdiz Iturri JB, García Rio F, Güell Rous R, Morante Velez F, et al. Oxigenoterapia continua domiciliaria. Arch Bronconeumol. 2014;50:185–200.