There has been a growing interest in recent years in the extraosseous effects of vitamin D.
In this article, we review the physiology of vitamin D, the physiopathological effects associated with vitamin D deficit and the available evidence on its etiopathogenic role in respiratory diseases. Given the pleiotropic actions of vitamin D, it is biologically plausible that the deficit of this vitamin could play a pathogenic role in the development of various respiratory diseases. However, the many epidemiological studies that have shown an association between low vitamin D levels and a higher risk of developing various respiratory diseases, or a poorer prognosis if they do appear, were unable to show causality. Post hoc analyses of some clinical trials, particularly in chronic obstructive pulmonary disease (COPD) and asthma, appear to suggest that some patient subtypes may benefit from correction of a vitamin D deficit. In this respect, it would be interesting to determine if the interindividual differences found in the effect of vitamin D deficit and responses to correcting this deficit could be explained by the genetic variants involved in vitamin D metabolism. Ultimately, only appropriately designed clinical trials will determine whether 25-OH D supplements can prevent or improve the course of the various respiratory diseases in which an epidemiological association between prognosis and vitamin D deficit has been described.
En los últimos años existe un creciente interés por las acciones extraóseas de la vitamina D.
En este artículo revisamos la fisiología de la vitamina D, los aspectos fisiopatológicos asociados a su déficit y la evidencia existente sobre su papel etiopatogénico en enfermedades respiratorias. Teniendo en cuenta las acciones pleiotrópicas de la vitamina D, existe plausibilidad biológica sobre un potencial papel patogénico del déficit de esta vitamina en el desarrollo de diversas enfermedades respiratorias. Sin embargo, los numerosos estudios epidemiológicos que han encontrado asociación entre niveles bajos de vitamina D y mayor riesgo de desarrollar diversas enfermedades respiratorias o de conllevar un peor pronóstico no permiten demostrar causalidad. Los análisis post hoc de algunos ensayos clínicos, especialmente en enfermedad pulmonar obstructiva crónica (EPOC) y asma, parecen demostrar que ciertos subtipos de pacientes podrían beneficiarse de la corrección del déficit de vitamina D. En este sentido, resultará interesante averiguar si las variantes genéticas implicadas en el metabolismo de la vitamina D pueden explicar las diferencias interindividuales encontradas en cuanto al efecto del déficit de vitamina D y la respuesta a su corrección. En último término, solo los ensayos clínicos adecuadamente diseñados permitirán determinar si los suplementos de 25-OH D pueden tener un efecto preventivo o mejorar la evolución de las distintas enfermedades respiratorias en las que se ha descrito asociación epidemiológica entre su pronóstico y el déficit de esta vitamina.
Recent years have seen a growing interest in extraosseous actions of vitamin D. In this article we review the physiology of vitamin D, the pathophysiological aspects associated with its deficit and the evidence of its pathogenic role in respiratory diseases.
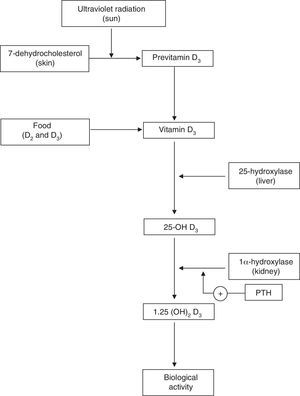
Physiology of Vitamin DVitamin D plays a key role in calcium homeostasis and bone metabolism (Fig. 1).1 The most important form of vitamin D (cholecalciferol or vitamin D3) is synthesized in the skin from 7-dehydrocholesterol, and it is also found in certain foods. Vitamin D obtained through skin synthesis or diet is biologically inactive. Activation requires a first hydroxylation in the liver for conversion to 25-hydroxy-vitamin D (25-OH D), the levels of which reflect vitamin D deposits the body; 25-OH D undergoes a second hydroxylation, mainly in the kidney, to exert its biological action.1–3 Renal production of 1,25 (OH)2 D is tightly regulated by the parathyroid hormone (PTH) and serum levels of calcium and phosphorus.4 Binding of 1,25 (OH)2 D to its receptor in the cell nucleus, predominantly in the small intestine, kidney and bone, among other tissues,1–3 stimulates the intestinal absorption of calcium and phosphorus from the diet5,6 and promotes renal reabsorption of calcium.2 Inadequate mineralization of the collagen matrix associated with vitamin D deficiency causes osteomalacia8 and rickets.7
Optimal Levels, Prevalence of Deficiency and Requirements of Vitamin DAn intense debate is currently ongoing regarding the optimal levels of vitamin D and the definition of vitamin D1,2 deficiency. Most authors define vitamin D deficiency as serum 25-OH D<20ng/ml,1,3,9 and insufficiency between 20ng/ml and 30ng/ml. Deficiency and insufficiency of vitamin D are thought to affect more than half of the general population,10 representing an emerging health problem throughout the world.11 In our country, a third of the Spanish population are at risk of developing vitamin D dficiency.12–14
Regarding the requirements for vitamin D for maintaining bone metabolism in adult general population, the recommended dose is 800IU/day–1000IU/day, although greater daily doses have been suggested (up to 2000IU/day) in elderly subjects with osteoporosis or steroid treatment and vitamin D deficiency.15 With regard to the possible deleterious effects of vitamin D supplements, toxicity has not been demonstrated in patients with levels of 25-OH D below 100ng/ml.9
Extraosseous Effects of Vitamin D and Its Role in Other ConditionsThe ubiquitous distribution of vitamin D receptor (VDR) suggests that this vitamin plays roles unrelated to mineral metabolism and that its receptors can be activated by other ligands.1 Thus, 1-α hydroxylase activity that would facilitate activation of 25-OH D has been described in numerous extrarenal cells. Vitamin D binding to its specific receptor regulates the transcription of 200 genes involved in the regulation of cell growth and maturation, inhibiting the renin-angiotensin axis, and angiogenesis, insulin secretion and sensitivity to it. This has given rise to the hypothesis that there is a potential etiopathogenic role in various extraosseous diseases.3,16 In this regard, epidemiological associations between vitamin D deficiency and certain cancers, diabetes, cardiovascular and autoimmune diseases, among others, have been described.1,2,15 There is abundant evidence supporting the role of vitamin D in the immune system which, in turn, would determine a possible effect on the development of respiratory diseases. It should be noted that almost all cells mediating adaptive and innate immune response, including activated CD4 and CD8 lymphocytes, B lymphocytes, neutrophils, and antigen-presenting cells such as macrophages and dendritic cells, have vitamin D receptors that act as powerful inmunomodulators,17 justifying a lower innate immune response in monocytes and macrophages with serum levels of 25 OH D<20ng/ml.18 In theory, vitamin D-dependent macrophage activation would neutralize microorganisms involved in respiratory infections and promote an immunomodulatory effect of the adaptive response, thus preventing its deleterious effect on the host.16 In line with these data, an epidemiological association between vitamin D deficiency and an increased risk of certain respiratory infection has been observed.19,20 This mechanism would also help vitamin D to protect against respiratory infections that can trigger the worsening of asthma.21 Furthermore, the regulatory effect of this vitamin on immune cells involved in the pathogenesis of asthma could have a beneficial effect on bronchial hyperreactivity.22
It has been suggested that the increased susceptibility to respiratory infections in subjects with vitamin D deficiency would promote chronic inflammation in the airways, which plays a central role in the pathogenesis of chronic obstructive pulmonary disease (COPD).23 Interestingly, 1,25-OH D is a potent inhibitor of dendritic cell maturation, by decreasing the expression of class II molecules of the major histocompatibility complex and costimulation, thus reducing the production of proinflammatory cytokines such as interleukins2,12,23 and interferon gamma.24 Vitamin D deficiency contributes to the pathophysiology of COPD through its effects on airway smooth muscle and lung remodelation by its actions on fibroblast proliferation, collagen synthesis and modulation of matrix metalloproteinase levels.25 In addition, osteoporosis-induced vitamin D deficiency increases the risk of rib fractures and vertebral compressions, especially in severe stages of COPD, thus reducing FEV1 and FVC.26,27 The effect of vitamin D on skeletal muscle has suggested the hypothesis that the deficit contributes to the respiratory muscle weakness observed in advanced stages of COPD.22
The relationship between vitamin D and other respiratory diseases, based on observational studies and clinical trials, is reviewed below.
Chronic Obstructive Pulmonary DiseaseRecent studies have shown that, compared with smokers without COPD, patients with advanced COPD frequently have vitamin D deficiency, with a prevalence of between 33% and 77%, and higher in advanced stages of the disease.28–30 Factors that explain this include the alteration of the cutaneous synthesis of vitamin D due to age and the toxic effects of tobacco, low exposure to sunlight, increased catabolism of vitamin D by glucocorticoids, its sequestration in adipocytes, reduced intestinal absorption and poor hepatic and renal activation of vitamin D precursors, among others.24
Several studies show a decrease in the daily dietary intake of vitamin D in patients with COPD,31 especially the elderly.32 Although increased intake of vitamin D appears to be associated with better lung function and reduced prevalence of COPD,33 a recent longitudinal study in current smokers with mild to moderate COPD found no relationship between low basal levels of vitamin D and deterioration of respiratory function34 or the risk of exacerbations.35 Another recent prospective cohort study showed no relationship between baseline serum vitamin D levels and mortality in a group of patients with moderate to severe COPD.36 However, Janssens et al.29 found that circulating serum 25-OH D levels were significantly correlated with FEV1 in COPD patients compared with healthy smokers. A limitation of any of the above studies is that the seasonal variation and latitude that modify exposure to sunlight and consequently the levels of vitamin D were not taken into account. In this regard, a more robust association has been described between vitamin D levels and respiratory function during winter.37
It has been suggested that vitamin D deficiency, because of the secondary hyperparathyroidism it induces, is associated with the development of osteoporosis, and proper supplementation reduces the risk of osteoporotic fractures.2,3 Several observational studies have shown correlation between bone mineral density, severity of COPD, and exercise capacity, also demonstrating association between vitamin D levels and oxygen saturation.38,39 Another interesting observational study confirmed a higher prevalence of osteoporosis and osteopenia in patients with COPD compared with healthy former smokers.40
The disparity of results on the relationship between serum 25-OH D levels and lung function in COPD patients has led to the hypothesis that there are subgroups of COPD patients with different genetic predisposition for developing vitamin D deficiency. Thus, the association between serum levels of vitamin D and the severity of COPD has been described as particularly significant in patients carrying certain gene variants of the vitamin D transport protein, which in turn is independently associated with increased risk of COPD.29,41 Moreover, other polymorphisms of the vitamin D binding protein have been reported to reduce the risk of developing COPD42 or presenting exacerbations of this disease.43 By contrast, no link between VDR polymorphisms and risk of developing respiratory infections in patients with COPD has been found.44
A recent clinical trial analyzed the effect of high doses of vitamin D on the incidence of exacerbations in COPD patients. No significant differences were found in time to first exacerbation or first hospitalization due to COPD, the annual rate of exacerbations or mortality.45 However, a post hoc analysis showed that, among patients with severe vitamin D deficiency (25-OH D<10ng/ml), the annual rate of COPD exacerbations was reduced by 43% and that, in this subgroup of patients, treatment with vitamin D was associated with increased phagocytic capacity of monocytes. It should be noted that administration of vitamin D, regardless of baseline levels or the presence of genetic polymorphisms of the vitamin carrier protein, may have minimized the potential beneficial effect of supplementation. A reanalysis of the data from this study revealed that supplements of vitamin D are associated with a significant improvement in inspiratory muscle strength and oxygen consumption.46 However, another pilot study of vitamin D supplementation in COPD showed no improvement in short-term physical performance.47 The limited availability of clinical trials and their limited sample size suggest the need for larger studies with longer follow-up periods, aimed at analyzing the impact of the administration of vitamin D in patients with demonstrated vitamin deficiency.48 A study is being conducted (VidiCo, NCT00977873) which analyzes the effect of vitamin D administration on the risk of exacerbations in patients with less severe COPD. In addition, the Lung VITAL substudy will investigate the effect of daily administration of 2000IU of vitamin D on the risk of exacerbations in patients with COPD.
AsthmaIt has been suggested for some years that vitamin D deficiency has a pathogenic role in both the development and the course of asthma.49 Evidence from population studies shows a higher prevalence of vitamin D deficiency in children with asthma compared with that in controls.50 In addition, vitamin D deficiency is associated with a higher probability of severe exacerbations in children with mild to moderate persistent asthma.51 Children with vitamin D deficiency also have reduced lung function, increased bronchial reactivity to exercise52 and an increased need for inhaled corticosteroids.53 In contrast, high levels of vitamin D have been associated with improved lung function, reduced bronchial hyperreactivity and better response to glucocorticoids.54 Several studies have examined the relationship between prenatal exposure to vitamin D and the development of asthma in childhood, with greatly varying results.55–57 A recent study has found that asthmatic children with vitamin D deficiency and treatment-resistant severe asthma have increased bronchial smooth muscle and poorer control of the disease.58
Airway InfectionsThe higher incidence of respiratory infections during winter, coinciding with a lower sun exposure and suboptimal serum levels of vitamin D, has served as the basis for a hypothetical relationship between vitamin D levels and increased susceptibility to respiratory infections. As previously mentioned, vitamin D has a modulating action on various cellular and molecular mediators involved in the inflammatory response to various stimuli. The association between expression of VDR gene polymorphisms such as Fok-I and Taq-I and an increased risk of airway infections is interesting.59 Although an association between vitamin D levels and incidence of upper respiratory tract infection was observed in the NHANES III study, especially in patients diagnosed with COPD and/or asthma,60 a recent study concluded that supplementation of vitamin D3 does not reduce the incidence of respiratory upper airway infections.61 However, it should be noted that the inclusion in this trial of patients who had no vitamin D deficiency at baseline may have attenuated the effect of supplementation.
TuberculosisSeveral studies have shown that patients with tuberculosis have decreased serum levels of vitamin D compared with healthy controls.62,63 As in other respiratory infections, vitamin D plays an important role in the immune response against Mycobacterium tuberculosis infection. Several polymorphisms have been identified in VDR and vitamin D binding protein that influence the risk of developing tuberculosis, and also in response to treatment.64 Administration of vitamin D in a randomized clinical trial increased the proportion of sputum conversion and radiological improvement compared to placebo administration.65
Cystic FibrosisSeveral studies have shown that, despite the contribution of supplements, patients with cystic fibrosis have low levels of 25-OH D, and patients with cystic fibrosis and vitamin D deficiency require greater supplementation of vitamin D.66,67 However, a recent meta-analysis concluded that there is no evidence of benefit or harm with vitamin D supplementation in patients with cystic fibrosis.68
Lung CancerAlthough experimental data suggest a suppressive effect of vitamin D on the development of lung cancer, observational studies show controversial data on the relationship between vitamin D levels and risk of lung cancer.69 One of these studies found that vitamin D deficiency was a risk factor for lung cancer only in women and young patients.69 In contrast, another study showed no relationship between vitamin D levels and overall survival of patients with lung cancer.70 Although a synergistic effect of vitamin D to chemotherapy for lung cancer has been described, a clear beneficial effect of vitamin D in such malignancies has not been demonstrated.71,72 However, it is interesting that increased VDR expression in lung cancer is associated with improved survival, attributed to a lower proliferative and cell cycle arrest in G1 phase.73,74
Interstitial Lung DiseaseThe participation of vitamin D in fibroproliferation in response to inflammation and damage to the bronchial epithelium has led to the hypothesis that it plays a role in the development of interstitial lung diseases (ILD).75 In this regard, a high prevalence of vitamin D deficiency in patients with ILD has been described, particularly those with connective tissue diseases; the associated reduction in lung function may suggest that this vitamin has a role in the pathogenesis of ILD.76
SarcoidosisIn the case of sarcoidosis, vitamin D and its metabolites have a negative effect, due to possible induction of hypercalcemia that occurs in approximately 5% of the patients.77,78 In patients with sarcoidosis, elevated levels of 1,25 (OH)2 D have been observed to be associated with an increased need for chronic treatment and repeated cycles of immunosuppressants.79
Lung TransplantSeveral observational studies have shown a high prevalence of vitamin D deficiency in lung transplant recipients, with a direct relationship between low levels of vitamin D and reduced lung function, poorer outcomes and higher incidence of severe rejection phenomena.80,81 A recent study concluded that one-year mortality in patients who maintained vitamin D deficiency after transplantation was higher than in those who had normal levels of vitamin D, and vitamin D deficiency was associated with a higher incidence of acute rejection and infections.82
ConclusionsGiven the pleiotropic actions of vitamin D, there is some biological plausibility of a potential pathogenic role of the deficiency of this vitamin in the development of various respiratory diseases. However, the numerous epidemiological studies that have found an association between low levels of vitamin D and increased risk of various respiratory diseases or poor outcome do not allow causality to be established. The post hoc analyses in some clinical trials, especially in COPD and asthma, suggest that certain subtypes of patients would benefit from the correction of vitamin D deficiency. In this regard, it would be interesting to determine whether genetic variants involved in the metabolism of vitamin D may explain the interindividual differences found in the effect of vitamin D deficiency and response to correction. Ultimately, only properly designed clinical trials will determine whether supplements of 25-OH D may have a preventive effect or improve the development of the various respiratory diseases in which an epidemiological association between prognosis and the lack of this vitamin has been described.
FundingThis study has not received any funding.
Conflicts of InterestThe authors declare they do not have conflicts of interest related to the content of this manuscript.
Please cite this article as: García de Tena J, El Hachem Debek A, Hernández Gutiérrez C, Izquierdo Alonso JL. Papel de la vitamina D en enfermedad pulmonar obstructiva crónica, asma y otras enfermedades respiratorias. Arch Bronconeumol. 2014;50:179–184.