We describe the case of a 45-year-old male who was admitted with clinical signs of superior vena cava syndrome (SVCS). Physical examination showed collar of Stokes and extensive collateral circulation in the neck and anterosuperior thoracic region, as well as a large testicular mass. Fibrobronchoscopy revealed an endobronchial tumor, histopathologically diagnosed as seminoma, with the same characteristics as the testicular biopsy. Treatment was initiated with surgery, chemotherapy and radiotherapy, resulting in a major clinical improvement. We indicate the importance of considering SVCS as an entity related with less common neoplasms such as germ cell tumors.
Se describe el caso de un varón de 45años que ingresó con signos clínicos de un síndrome de vena cava superior (SVCS), destacando en la exploración edema en esclavina y gran circulación colateral en el cuello y en la región anterosuperior torácica, así como una gran masa testicular. La fibrobroncoscopia mostró una lesión endobronquial tumoral, cuyo diagnóstico histopatológico fue de seminoma, con las mismas características que la biopsia testicular. Se inició tratamiento con cirugía, quimioterapia y radioterapia, presentando gran mejoría clínica. Señalamos la importancia de considerar el SVCS como entidad relacionada con procesos neoplásicos menos frecuentes, como son los tumores germinales.
Superior vena cava syndrome (SVCS) is a rare condition that occurs in 7% of all cancers.1,2 Its etiopathogenesis is multifactorial and a few cases associated with testicular carcinoma3,4 have been reported, of which only one was endobronchial involvement.5 We report a case of SVCS with mediastinal and endobronchial involvement due to testicular carcinoma, which we consider a rare combination.
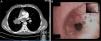
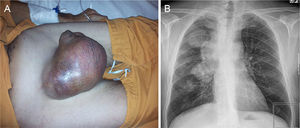
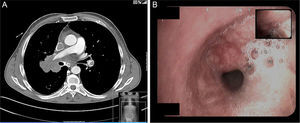
Case ReportA 45-year-old male, smoker of 56 packs/year, operated for cryptorchidism. He came to our clinic complaining of dyspnea on heavy exertion which had worsened to dyspnea on minimal exertion two weeks previously, accompanied by weight loss in the last year, and painless enlargement of the right testicle in the last 4 months. He also reported headache and swelling of the right hemithorax, neck and face. Notable findings on physical examination were central cyanosis, exophthalmos, conjunctival injection, jugular ingurgitation, facial and upper extremity edema and collateral circulation in the neck and right hemithorax. Examination of his genitals revealed a right petrous, painless, irreducible testicular mass measuring 16cm×14cm, and left inguinal adenopathy (Fig. 1A). The chest X-ray showed displacement of the right paratracheal line due to a mediastinal mass, and a 4.6cm×4cm mass in the right upper lobe (Fig. 1B). Laboratory tests revealed mild leukocytosis, mild normochromic, normocytic anemia and elevated D-dimer (5270μg/l). Computed tomography angiography showed a 7cm×7.6cm mediastinal mass involving the right pulmonary hilum, surrounding the right main bronchus, the bronchus intermedius and middle lobe, and displacing the superior vena cava, which was found to be thrombosed, and the right main pulmonary artery, which was extensively thrombosed. Thrombosis of the left lower pulmonary artery was also observed (Fig. 2A). There was collateral circulation through the azygos system secondary to vena cava thrombosis. A 7.5cm×8cm polylobulated mass was observed in the retroperitoneum, encompassing the inferior vena cava, with left paraaortic adenopathies in right common iliac, common femoral and right inguinal nodes, in addition to a 13cm×12.7cm mass in the right inguinoscrotal canal.
Fiberoptic bronchoscopy revealed hypervascularization and displacement of the lower and middle third of the trachea and a whitish vascularized tumor in middle lobe bronchus (Fig. 2B).
Testicular ultrasound showed a normal left testis, displaced by a 15cm×13cm×12.5cm highly vascularized solid mass in the right hemiscrotum.
Respiratory function tests showed FVC: 3690ml (76%); FEV1: 2840ml (73%); FEV1/FVC: 73; TLC: 5760ml (75%); RV: 2280ml (95%). Arterial blood gases (FiO2 0.21), pH: 7.38; pCO2: 39mmHg; PO2: 68mmHg; PO3−: 23mmol; D(A-a)O2: 33mmHg.
Tumor markers were as follows: β-HCG: 13mU/ml; AFP: 1.93ng/ml; LDH: 3679U/l; β2-microglobulin: 1.3ml/l; CA-19.9: 2U/l; CA-15.3: 14.5U/l; CEA: 0.81ng/ml; CA 125: 9.46U/ml.
Testicular biopsy was compatible with extensively necrotic seminoma. Bronchial biopsies were also compatible with primary tumor metastasis. The immunohistochemical pattern showed placental alkaline phosphatase, positive c-Kit (CD117), focal positive vimentin, and negative keratin, EMA, CD30, and p63. The definitive diagnosis was stage IIIC seminoma (pT4N3M1b) with bronchial metastases, superior vena cava syndrome and pulmonary thromboembolism. Following anticoagulation, dexamethasone and emergency radiotherapy to a total of 110Gy, clinical symptoms improved in a week, with nearly complete resolution of collateral circulation. Orchiectomy was deferred for 2 months. The patient received 4 cycles of cisplatin plus etoposide on an outpatient basis.
DiscussionTesticular carcinoma has an annual incidence of 6.3 cases per 100000 inhabitants and accounts for 2% of total cancers.3 It is considered the most common malignant neoplasm in men aged 15–34 years, although seminoma is the least aggressive type.3–5 The risk factors are cryptorchidism, Klinefelter's syndrome, hypotrophic testis or infertility.3 Pulmonary metastases occur in 15% of seminomas, although endobronchial involvement is rare and has a worse prognosis.3–5
SVCS was first described in 1757 by William Hunter in a patient with a syphilitic aneurysm of the aorta, and in 1954 Schechter reviewed 274 cases, 40% secondary to syphilitic aneurysms or tuberculous mediastinitis.1,2 These diseases are now rare and the main causes are malignancies,1–3 among which the most important are bronchogenic carcinoma (75%–80%), lymphoma (12%), metastases (9%), germ cell carcinoma (3%), thymoma (2%) and mesothelioma (1%).1,2 The etiopathogenesis of SVCS associated with tumors is influenced by direct compression, fibrosis secondary to thrombosis or intimal inflammation due to direct tumor infiltration or invasion.1 Because the superior vena cava is located in a non-distensible space within the mediastinum, drainage from the head, neck, upper extremities and the upper part of the chest is compromised, so that venous flow is redistributed through the azygos-hemiazygos system, internal mammary, paraspinal, esophageal and subcutaneous veins, causing the characteristic collateral circulation and facial and upper extremity edema.2,4 Other benign causes of SVCS are endothoracic goiter, mediastinal fibrosis, aortic aneurysm, sarcoidosis, Behçet's syndrome, histoplasmosis, tuberculosis, syphilis, or iatrogenesis (implantation of a pacemaker or central venous catheter).1,2
If obstruction is above the azygos vein, clinical symptoms are less obvious. However, obstruction below the azygos vein gives rise to extensive collateral circulation, ascites and even edema in the lower extremities. The typical symptoms of SVCS consist of headache, accompanied by somnolence, vertigo, cyanosis of the skin and mucous membranes of face, neck and upper extremities, conjunctival hemorrhage, facial and upper extremity edema, neck vein distention, collateral circulation in the chest wall, and even Claude-Bernard-Horner syndrome.1,2 Treatment is directed at the underlying cause, accompanied by general palliative measures, though in some emergency situations, such as brain edema, airway obstruction or decreased cardiac output, chemotherapy or radiotherapy may be required.1,2 If there is associated thrombosis, as in our case, anticoagulation should be initiated and thrombolysis or thrombectomy becomes occasionally necessary.1–4
The authors conclude that if SVCS is observed in young males, germ cell carcinoma should be considered in the differential diagnosis, even in the absence of such a florid presentation as was observed in this case.
Please cite this article as: Landete P, Chiner E, Sancho-Chust JN, Sánchez-Valverde MD, Pérez-Ferrer P, Bravo-Fernández R. Seminoma con afectación bronquial y síndrome de vena cava superior: una asociación excepcional. Arch Bronconeumol. 2014;50:201–203.