Respiratory morbidities of preterm infants can cause significant ventilatory impairment thus compromising the aerobic capacity in childhood and adolescence. Therefore, the present study was conducted to evaluate the aerobic capacity in school age preterm children with VLBW and its associated factors.
MethodsA cross-sectional study was conducted among preterm born with VLBW and term children, both aged 6–9 years. An individualized symptom-limited treadmill testing protocol performed aerobic capacity. Measured variables: oxygen pulse (PuO2), percentage of maximum heart rate for age (%HR max), tidal volume/inspiratory capacity ratio (TV/IC), oxygen consumption (VO2) peak, and the ratio of the anaerobic threshold of gas exchange to the predicted percentage of maximum VO2 (VO2@LA/%VO2 max.pred.) were compared between groups. Univariate and multiple linear regression analyses were used to determine the factors associated with aerobic capacity.
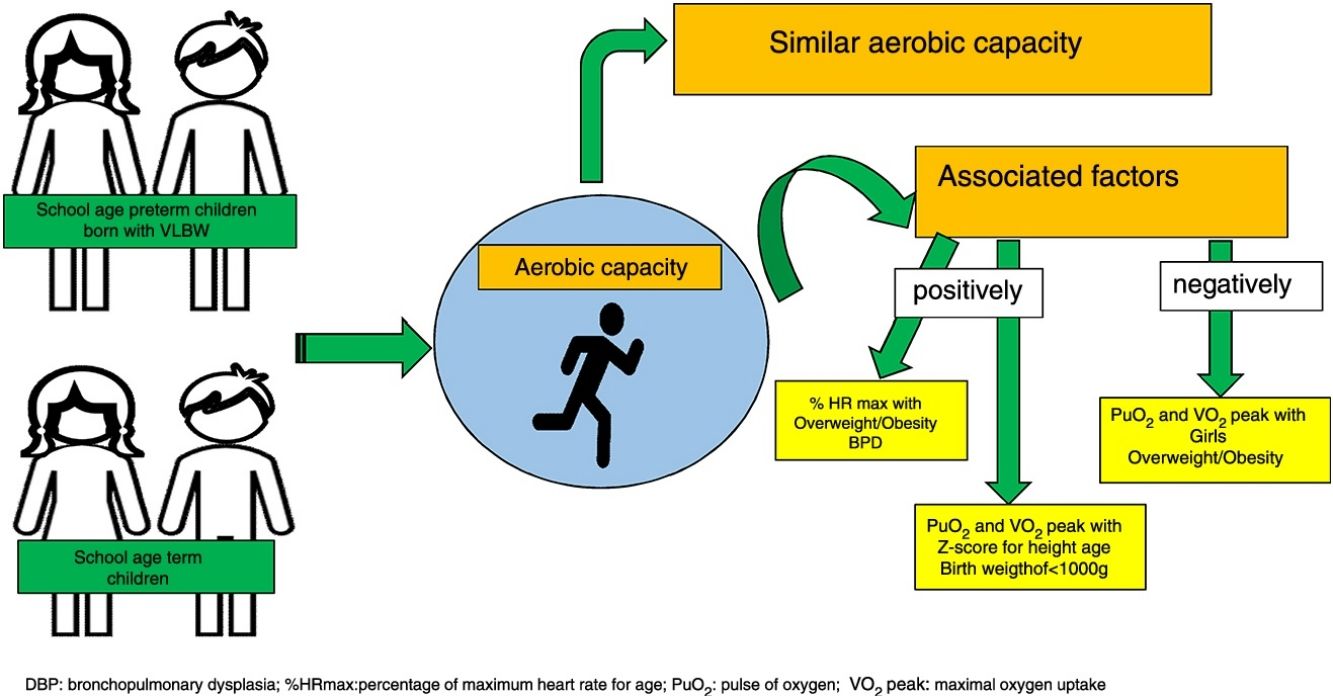
ResultsThirty-four preterm and 32 term children were included. Similar VO2 peak and the other variables were observed. The development of bronchopulmonary dysplasia (BPD) and being obese/overweight was positively associated with %HR max. The Z-score for height/age and birth weight <1000g was positively associated with PuO2 and peak VO2, and negatively associated with overweight/obesity and female sex.
ConclusionsAerobic capacity was similar in both groups. Sex, development of BPD, birth weight <1000g and factors related to body growth, such as Z-score for height/age and overweight/obesity, were associated with aerobic capacity in preterm children with VLBW.
Las enfermedades respiratorias de los niños prematuros pueden causar importantes impedimentos ventilatorios que comprometen la capacidad aeróbica en la infancia y en la adolescencia. El presente estudio se llevó a cabo para evaluar la capacidad aeróbica de niños prematuros en edad escolar de muy bajo peso al nacer (BPN) y los factores asociados.
MétodosSe llevó a cabo un estudio transversal con niños prematuros de muy BPN y con niños a término, ambos grupos con edades comprendidas entre los 6 y 9 años. Las siguientes variables se compararon entre los 2 grupos: el pulso de oxígeno (PuO2), el porcentaje de frecuencia cardíaca máxima (%FC máx.), la relación entre el volumen corriente y la capacidad inspiratoria (TV/IC), el consumo pico de oxígeno (VO2) y la relación entre el umbral anaeróbico de intercambio de gas y el porcentaje estimado de VO2 máximo (VO2@LA/%VO2 máx. pred.). Se llevaron a cabo análisis de regresión lineal univariante y multivariante para determinar los factores asociados con la capacidad aeróbica.
ResultadosSe incluyeron 34 niños prematuros y 32 niños a término. Se registraron valores similares de VO2 pico y de otras variables. El desarrollo de displasia broncopulmonar (BPD) y de obesidad/sobrepeso mostró una asociación positiva con el %FC máx. El Z-score para la altura/edad y el peso al nacer <1.000g se asoció positivamente con la SaO2 y el VO2 y negativamente con el sobrepeso/obesidad y el sexo femenino.
ConclusionesLa capacidad aeróbica fue similar entre los 2 grupos. El sexo, el desarrollo de BPD, peso al nacer <1.000g y factores relacionados con el crecimiento corporal, tales como el Z-score para la altura/edad y para el sobrepeso/obesidad se asociaron con la capacidad aeróbica en niños prematuros de muy BPN.
Respiratory morbidities remain the most common problem among preterm infants1 despite the recent improvements in postnatal care.2 Among respiratory morbidities, bronchopulmonary dysplasia (BPD) is the most important impairment and its pulmonary structural alterations and ventilatory and gas exchange limitations could decrease muscle strength3–5 and then, functional exercise capacity in early childhood and adolescence.6,7
During exercise, one of the main factors that determine exercise capacity is the maximum oxygen uptake (VO2 peak). Studies on adults and adolescents born extremely preterm have shown that peak VO2 values may be within the normal range.4,8 However, some authors have reported that decreased pulmonary diffusion capacity or altered pulmonary function may compromise the aerobic capacity in preterm children and adolescents, with a decreased peak VO2 or distance covered being observed on the cardiopulmonary exercise test (CPET) that is performed on a treadmill or cycle ergometer.8–10 Hence, the actual aerobic capacity and the associated factors with it in school age preterm children with very low birth weight (VLBW) remain controversial.
Therefore, the present study was conducted to evaluate the aerobic capacity in school age preterm children with VLBW and its associated factors.
MethodsA cross-sectional study was conducted on two groups of 6–9-year-old children of both sex, recruited between February 2013 and April 2015. The preterm group consisted of children born with gestational age of <37 weeks and birth weight of <1500g, accompanied at the multidisciplinary outpatient follow-up clinic of the institution. Term group consisted of children born with gestational age of ≥37 weeks, both sexes, without respiratory impairment or outpatient follow-up, and being relatives or friends of premature children attended in our outpatient clinic. Children with congenital malformations; neuromuscular diseases; neurological, visual, and/or hearing disorders; and/or other problems that may lead to difficulties on the execution of the test were excluded. Children with signs or symptoms of acute or chronic respiratory disease in the last 2 weeks or those with abnormal hemodynamic condition at rest were also excluded.11
This study followed the declaration of Helsinki. The Ethics and Research Committee of the Institution approved this study (Protocol: 173.275/12). The consent form was signed by the parents or guardians of the children, and the assent form was signed by the children.
Included children were evaluated according to a clinical questionnaire answered by their parents, and anthropometric variables were evaluated before cardiopulmonary exercise test. Additionally, the clinical records of preterm group during the neonatal period and their clinical status after discharge from the neonatal unit were also collected. In the term group, neonatal data were obtained from the birth card complemented by an interview with parents and/or legal guardians conducted by the researcher. The socioeconomic status and frequency of weekly physical activity of the child were evaluated through a questionnaire answered by the parents or guardians on the scheduled day of the test.12 The child's Body Mass Index (BMI) during the recruitment was determined according to the recommendations of the World Health Organization.13
A cardiopulmonary exercise test (CPET) and a symptom-limited treadmill test with a rapid incremental protocol and individualized increase in load was used to evaluate the aerobic capacity.14 The slope was calculated individually15 based on the fixed velocity of 3.5mph (children aged between 6 and 7 years) and 4.0mph (those aged between 8 and 9 years) and predicted maximum VO2 (VO2 max). For each age group, VO2 max was calculated according to child's sex and weight.16
Prior to the test, the child remained seated for 5min, and then vital signs (blood pressure, heart rate, and peripheral oxygen saturation) and degree of dyspnea were evaluated. The non-rebreathing valve face mask (Hans Rudolph, Kansa) of the gas analyzer (K4b2, Cosmed, Pavonadi Albano, Itália) was placed on the face of the child, and the device analyzer was placed in front of the treadmill in order not to impose a workload during the CPET. The gas analyzer was previously calibrated at the beginning of the tests according to the manufacturer's recommendations.
The CPET was started with 3-min warm-up phase17 maintaining the speed of 1.0mph without grade and during the last minute, the speed was raised according to the child's age. During the exercise phase, the grade was increased by 2% every minute until a maximum grade of 10%. If at this grade the child did not meet the maximum effort criteria,14,18 the speed was increased by 0.5mph every minute until meeting the interruption criteria. In the recovery phase, the child remained walking for 5min in the warm-up speed and grade.
During the test, expired gases were collected breath-by-breath, and data recording was filtered every 15s.19 Variables were analyzed for two periods: at rest and at the peak of the CPET, considering the values during the last 15–30s at the end of the test, based on arithmetic means.
The anaerobic threshold (AT) was obtained using the V-slope method16 after the concordance of two previously trained observers. The interpretation of anaerobic threshold of gas exchange (VO2@LA) was obtained based on the relationship between anaerobic threshold of gas exchange and percentage of maximum oxygen consumption predicted according to age (VO2@LA/% VO2 max.pred.).
To assess the aerobic capacity, the following cardiovascular, respiratory, and metabolic variables were analyzed: oxygen pulse (PuO2) obtained by the ratio ΔFC/ΔVO2; %HR max; tidal volume and inspiratory capacity ratio (TV/IC peak); VO2 peak, and the relationship between anaerobic threshold of gas exchange and percentage of maximum oxygen consumption predicted according to age (VO2@LA/%VO2 max.pred.).16,19,20
In order to consider the maximum CPET, the child was required to meet three of the following criteria: maximum HR ≥90% of the predicted HR according to age; signs of exhaustion, gaseous exchange ratio (R) of >1.0, and VO2 maximum of ≥85%.14,18 In addition, CPET was discontinued if some of the following intercurrences were identified: request for discontinuation by the child, failure of the monitoring device, pulse oxygenation decrease of ≥4% compared to the resting phase.16,21
The same protocol was used in all children, and a pulmonologist and/or pediatrician was present in the laboratory during the tests, together with the researchers of the study.
Statistical analysisSample size (G-Power 3.0.10) was calculated according to VO2 difference of 7.3mL/kg/min14 between groups, showed the need of 22 participants in each group, assuming an alpha error of 0.05 and beta of 0.80.
Numerical variables were compared using Student's t test (normal distribution) or Mann–Whitney test (non-normal distribution). Categorical variables were compared using the chi-square test or Fisher's exact test. The normality of variables was evaluated using the Kolmogorov–Smirnov test.
Regarding the analysis of factors associated with children's aerobic capacity, univariate and multivariate linear regressions were used considering the cardiovascular, respiratory, and metabolic variables related to an individual's aerobic capacity as dependent variables. Variables with statistical (p<0.2) and clinical significance in the univariate regression were included in the multiple linear regression models, excluding collinear variables in the same model. Statistical analyses were performed using the SPSS for Win/v.17.0 program (IBM SPSS Statistics, Somers, NY), and p<0.05 was considered statistically significant.
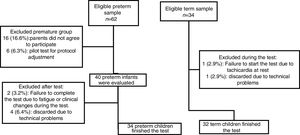
ResultsDuring the study period, 95 preterm children were attended in our outpatient clinic, however 33 (34.7%) of them did not meet inclusion criteria. Then, 62 preterm children were eligible and 34 term children (relatives or preterm children's friends) were invited to the study but at the end of the study 34 preterm and 32 term children finished the exercise test (Fig. 1).
The 22 (35.5%) eligible preterm children (but not included) were compared to 34 included preterm children, and no difference in the demographic and clinical characteristics was observed.
The mean birth weight and gestational age were 3072±609g and 38.5±1.4 weeks in the term group, and 1131±228g and 29.8±2.5 weeks in the preterm group. Clinical and demographic characteristics of both groups were similar, except for the lower Z score height for age and an increased need for hospitalization in the preterm group (Table 1).
Clinical and demographic characteristics of the sample.
| Preterm group (n=34) | Term group (n=32) | p value | |
|---|---|---|---|
| Age (years)a | 8.0±1.0 | 7.9±1.1 | 0.773 |
| Female (%)c | 17 (50.0%) | 14 (43.8%) | 0.631 |
| Baseline weight (kg)a | 27.2±6.5 | 28.4±7.6 | 0.499 |
| Z score weight for agea | 0.14±1.44 | 0.43±1.26 | 0.389 |
| Height (cm)a | 125.7±7.7 | 128.3±9.2 | 0.211 |
| Z score height for agea | −0.35±1.17 | 0.24±0.96 | 0.032 |
| BMI (kg/cm2)a | 17.0±2.6 | 17.0±2.8 | 0.944 |
| BMI (Z-score)b | 0.44±1.3 | 0.40±1.2 | 0.710 |
| Overweight, n (%)d | 8 (23.5%) | 4 (12.5%) | 0.342 |
| Obesity, n (%)d | 5 (14.7%) | 4 (12.5%) | 1.000 |
| Hospitalization after NICU discharge, n (%)d | 14 (45.2%) | 5 (15.6%) | 0.014 |
| Physical activity (h/week)b | 3.3±1.4 | 3.8±1.5 | 0.190 |
BMI: body mass index.
During the CPET, all children reached the maximum speed and grade recommended in the protocol, and in some cases, the speed was also increased meeting all the criteria for the maximum CPET. Despite this, the speed variation of the treadmill was similar between groups (preterm group: 23.5±13.5% vs. term: 26.0±9.6%, p=0.312). All children presented sinus rhythm on the electrocardiogram from the beginning until the recovery phase.
Aerobic pattern was similar between groups according to cardiovascular, respiratory, and metabolic variables, as described in Table 2.
Cardiovascular, respiratory, and metabolic variables.
| Preterm group (n=34) | Term group (n=32) | p value | |
|---|---|---|---|
| PuO2 (mL/bpm)a | 6.0±1.4 | 6.2±1.4 | 0.535 |
| %HR maxb | 87.9±4.1 | 86.1±5.9 | 0.244 |
| TV/IC peak (L)b | 0.615±0.146 | 0.592±0.131 | 0.735 |
| VO2 peak (mL/min)a | 1085±273 | 1102±277 | 0.798 |
| VO2 peak (mL/kg/min)a | 40.4±6.5 | 39.5±6.4 | 0.541 |
| VO2@LA/%VO2 max.pred. (%)a | 67.8±18.1 | 69.0±10.7 | 0.738 |
PuO2: oxygen pulse, expressed by the VO2/HR ratio; HR: heart rate; %HR max: percentage of maximum heart rate of age; TV: tidal volume; IC: inspiratory capacity; VO2 peak: maximal oxygen uptake; VO2@LA: VO2 at anaerobic threshold; %VO2 max.pred.: percentage of predicted VO2 max. p value:
Multiple linear regression analyses for possible factors associated with aerobic capacity are shown in Tables 3–5. The %HR max was positively associated to the dependence of O2 for more than 28 days and overweight/obesity. Oxygen pulse was positively associated to Z score height/age and negatively to female sex. In the same way, VO2 peak (mL/kg/min) was negatively associated to female and overweight/obese but positively to birth weight < 1000 g.
Multiple regression analysis of factors associated with the percentage of maximum heart rate for age (%HR max).
| Beta | 95% CI | p | |
|---|---|---|---|
| Oxygen dependence at 28 days of life | 3.153 | 0.244 to 6.063 | 0.034 |
| Overweight/obese | 2.628 | −0.181 to 5.438 | 0.066 |
Controlled model for birth weight of <1000g, respiratory distress syndrome, oxygen dependence at 28 days of life, duration of mechanical ventilation, days of hospitalization, overweight/obese. Model significance, p=0.006, r2=0.165.
Multiple regression analysis of factors associated with oxygen pulse (PuO2).
| Beta | 95% CI | p value | |
|---|---|---|---|
| Female | −0.683 | −1.266 to −0.100 | 0.022 |
| Z score height for age | 0.712 | 0.447 to 0.977 | <0.001 |
Controlled model for sex, Z score height for age, and overweight/obese. Model significance, p<0.001, r2=0.338.
Multiple regression analysis of factors associated with VO2 peak (mL/kg/min).
| Beta | 95% CI | p | |
|---|---|---|---|
| Female | −4.326 | −0.337 to −3.111 | 0.003 |
| Birth weight of <1000g | 4.690 | 0.257 to 2.370 | 0.021 |
| Overweight/obese | −5.624 | −0.409 to −3.837 | <0.001 |
Controlled model for sex, Z score height for age, overweight/obesity, birth weight of <1000g. Model significance, p<0.001, r2=0.300.
The following respiratory and metabolic parameters, the relationship between VC/IC peak, VO2 in absolute values, and VO2 LA/% VO2 max.pred., did not show significant association in the univariate linear regression; thus, they were not included in the multiple regression analysis.
DiscussionThe present study showed that children aged between 6 and 9 years with VLBW had similar aerobic capacity to children born at term. Nevertheless, after adjusting the covariates, these parameters were associated with the history of bronchopulmonary dysplasia, birth weight of <1000g, sex and factors related to body growth, such as Z score height for age and overweight/obesity.
The results in the univariate analysis demonstrate that at school age, preterm children had an aerobic capacity similar to that of full-term children. Some hypotheses may explain this similarity as evidenced by the fact that the included preterm children were born after undergoing measures to reduce mortality and neonatal morbidity, such as the use of exogenous surfactant, antenatal corticosteroids, and gentle ventilation modes.22 These measures have certainly improved the development and lung growth of preterm infants, reducing the severity of pulmonary complications such as bronchopulmonary dysplasia.23 Another hypothesis would be the low frequency of physical activity of the children in both groups, which may have influenced their aerobic responses.24 These children had a very low physical activity (approximately 3.5h/week in both groups) when compared to the recommended physical activity for this group age (60min daily) to prevent sedentary lifestyle.24 Additionally, the final hypothesis is the possibility of these preterm children to be like adults with chronic obstructive pulmonary disease, which increases the work of respiratory muscles during exercise to maintain or reach their aerobic capacity, i.e., within the normal range.25
Although the results of physical capacity seemed similar between groups, other factors were important according to the multivariate regression analysis. The presence of bronchopulmonary dysplasia increased by 3% maximum heart rate for age. This association could be explained by reduced ventilatory reserve and decreased lung diffusion capacity in preterm children, which can be supplied by increased cardiovascular system demand during exercise, represented by the increase in the %HR max.2,10 Moreover, children with history of pulmonary disease during the neonatal period have greater degree of respiratory impairment. Maybe, if we have had a greater sample of preterm children with bronchopulmonary dysplasia, the difference in cardiopulmonary conditions could possibly be showed.
Other important neonatal variable was associated with physical capacity as lower weight of birth. Birth weight of <1000g increased the VO2 peak in approximately 4mL/kg/min. It is known that preterm infants, especially the extremely ones may present air trapping during exercise, which involves pulmonary mechanics in an indirect way.5 In the present study, premature birth or BPD were not associated with any ventilatory variable, such as VC/IC peak, however, we cannot affirm that preterm infants do not present ventilatory or pulmonary mechanics alterations during exercise, since these variables were not evaluated by spirometry, being a limitation of the present study.
In addition to factors inherent to prematurity, sex and factors related to body growth, such as Z-score for height/age and overweight/obesity seemed to be associated with cardiovascular and metabolic conditions during exercise. The nutritional status, more specifically, being obese or overweight, reduced the VO2 peak by approximately 6mL/kg/min and increased by about 3% %HR for age. Zwiren26 describes that the peak VO2 expectedly increases based on an individual's growth; however, when excessive body weight gain occurs, as in the case of overweight or obesity, the association may be negative because the percentage of body fat is higher than body growth.27 Our results are consistent with the study of Welsh et al.8 that studied preterm children aged between 10 and 11 years by cycloergometer test. They also showed that impaired body growth was associated with decreased aerobic capacity. Our results show the importance of a good monitoring of nutritional status, especially in relation to obesity and overweight during follow-up of these premature infants during childhood.
Another important factor that presented a positive association was oxygen pulse. Despite this association, it did not clinically alter the PuO2 behavior in these children. This association was expected as studies had demonstrated the association between increased VO2 peak and body growth. As already known, PuO2 is dependent on VO2, which were expected to increase together.26,28
Finally, the last factor associated with cardiovascular and metabolic parameters was sex, which demonstrated that being female reduced the PuO2 and VO2 peak (mL/kg/min). As previously demonstrated, PuO2 is dependent on the VO2 peak, as girls had lesser PuO2, may be associated with the lower VO2 peak presented.26,27
Ventilatory reserve expressed as TV/IC peak ratio is expected to be associated with some independent variables because of the presence of BPD or prematurity itself, as previously reported in the literature.2,4,8 This association was expected because preterm infants may present pulmonary hyperinflation, which would reduce the tidal volume and inspiratory capacity to exercise. Then, preterm infants in their study sample possibly did not present significant pulmonary sequelae due to BPD or prematurity per se.
Regarding metabolic parameters, absolute values of VO2 and VO2@LA/%VO2 max.pred. ratio4 studies had shown controversial results, which were expected to be not associated with preterm parameters.2,28,29
The varied results presented in our study and the literature can be explained by the inclusion criteria. Most studies included heterogeneous population4 and different types of protocols and ergometers that could also influence the results.4,9 Thus, including children with homogeneous age group and similar growth and developmental characteristics as in our study could increase the internal validity and reliability of the presented data, because studies have shown that sexual maturation may play an important role in the performance of exercise capacity in preterm children.30 Although the groups presenting statistical difference in the Z score height/age ratio, their mean values are within normal limits, showing the similarity of growth and development between groups. The application of an individualized CPET loading protocol in the treadmill also favored the validity of results, because this type of ergometer protocol can better identify the peak VO2 in children.17 Aside from this factor, controlling the weekly physical activity of children, which would be a possible confounding factor, increased the quality of results in this study.
The percentage of loss and the number of children that had not met criteria to participate to the study could be a limitation in this study and it is possible that if we had a larger sample we could reach more precise conclusions. However, the analysis of the main demographic and clinical characteristics comparing the included and not included preterm children showed no significant difference, especially in relation to variables that could be biased, such as BPD and VLBW. Another limitation was the inclusion of a convenience sample, excluding preterm children with severe motor and cognitive sequelae. Those with severe neurodevelopmental impairments are also possibly the extremely preterm ones and with higher occurrence of bronchopulmonary dysplasia, and thus they could show a decreased aerobic capacity. However, these patients cannot be included due to the characteristics required in the protocol.
ConclusionsPreterm children with VLBW at school age showed aerobic capacity similar to that of full-term children. However, the intensity of responses during the cardiopulmonary exercise test was associated with sex, BPD, lower birth weight and factors related to body growth, demonstrating the influence of neonatal factors and body growth on the aerobic capacity in these children.
FundingThis work was supported by the Brazilian Government Scholarship—CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, in which Sabrina Pinheiro Tsopanoglou was supported by as a postgraduate student.
AuthorshipTsopanoglou S.P., Davidson J., Dourado V.Z., Goulart A.L., Barros M.C.M., Santos A.M.N.: the conception and design of the study; acquisition of data; analysis and interpretation of data; drafting the article; revising it critically for important intellectual content and final approval of the version to be submitted.
Conflict of interestsThe authors declare no conflict of interests.
Sabrina Pinheiro Tsopanoglou was supported by the Brazilian Government Scholarship—CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, as a postgraduate student.