Osimertinib is an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) that has demonstrated efficacy against T790M-positive non-small cell lung cancer (NSCLC).1 NSCLCs with the T790M mutation are mostly adenocarcinomas, and whether osimertinib is effective against T790M-positive squamous cell carcinoma (SqCC) remains uncertain. Here, we report a case of acquired T790M-positive SqCC that responded to osimertinib therapy.
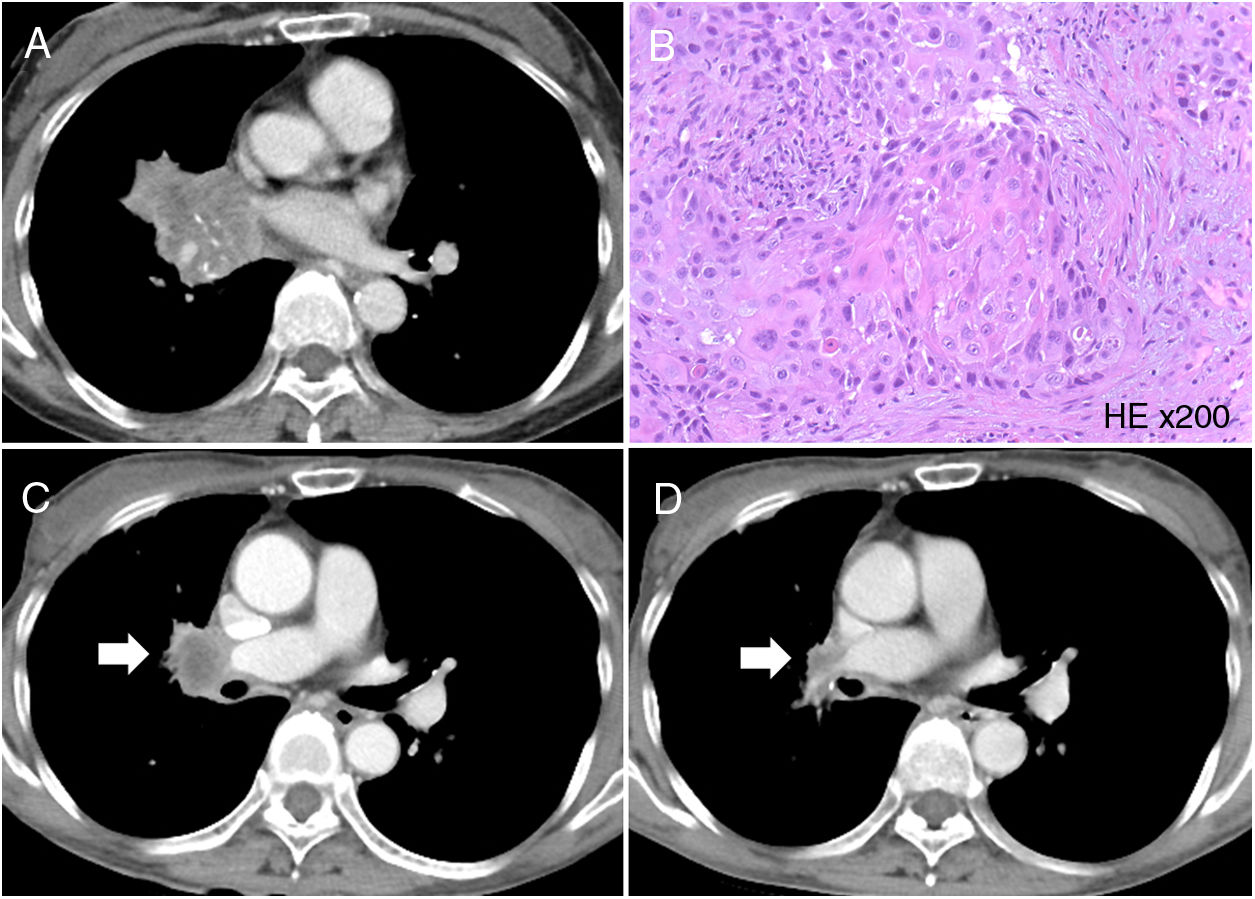
A 68-year-old woman arrived at our hospital with a chief complaint of prolonged productive cough. She was a never-smoker and had Parkinson's disease. She had been treated with deep brain stimulation and medication. However, her physical activity was relatively low. A chest radiography showed a mass in the right hilar region of the lower lungs, and a computed tomography (CT) scan showed a mass lesion in the right hilar region of the lower lobe of the right lung (Fig. 1A). The serum concentration of cytokeratin 19 fragment (CYFRA 21-1) was elevated (12.5ng/mL), and other tumour markers were within the normal ranges. Subsequently, she underwent bronchoscopy, and the biopsy specimen was pathologically examined. As a result, the tumour was diagnosed as SqCC (Fig. 1B). The specimen showed p63 positivity on immunohistochemical staining and EGFR mutation positivity (exon 19 deletion and exon 20 insertion). She underwent [18F]-fluorodeoxyglucose (FDG) positron emission tomography; results showed high FDG uptake in the right hilar lymph node and spleen; finally, her condition was diagnosed as primary lung SqCC, cT4N1M1b, stage IVA.
(A) Computed tomography (CT) scan showed a mass lesion in the right hilar region of the lower lobe of the right lung. (B) Haematoxylin and eosin staining of the biopsy specimen. Histopathological findings of the biopsy specimen showed squamous cell carcinoma (magnification 200×). (C) CT scan showed regrowth of the tumour (arrow). (D) The tumour showed shrinkage after osimertinib administration (arrow).
She was administered 150mg erlotinib daily. Four weeks later, the tumour lesions showed shrinkage, and the serum CYFRA 21-1 level was normalised. However, only three months later, the tumour showed regrowth, and the serum CYFRA 21-1 was elevated again (4.8ng/mL). As second-line treatment, chemotherapy using carboplatin and nab-paclitaxel was initiated. After the administration of two cycles of this regimen, the tumour shrunk, and the serum CYFRA 21-1 levels normalised. However, after four cycles, the tumour showed regrowth (Fig. 1C), and the serum CYFRA 21-1 levels elevated again (4.2ng/mL). The serum carcinoembryonic antigen and sialyl Lewis X-1 levels were not increased. A liquid biopsy was performed to detect EGFR mutations, and T790M and exon 19 deletion were detected.
As third-line treatment, 80mg osimertinib was administered daily. No particular adverse event was observed. After three weeks, the tumour showed shrinkage on CT scan (Fig. 1D), and the serum CYFRA 21-1 level normalised. After three months of osimertinib therapy, the tumour showed further shrinkage on CT scan. The patient is alive with no complaints or disease progression, and has continued osimertinib treatment for a total of 5 months.
In this case, we found that osimertinib was effective for a patient with acquired T790M-positive SqCC. To the best of our knowledge, only one case of a patient with acquired T790M-positive SqCC who showed response to osimertinib has been reported previously,2 along with other SqCC transformation cases.3,4 Osimertinib might be effective for T790M-positive SqCC; recently, osimertinib has been used as first-line therapy for EGFR mutation-positive NSCLC.5 It remains uncertain whether osimertinib is effective against T790M-negative EGFR mutation-positive SqCC. Further studies are required to evaluate the efficacy of osimertinib for T790M-negative EGFR mutation-positive SqCC.
EGFR mutations in SqCC should be examined, at least in never-smoker cases. SqCC with EGFR mutations are found at low frequencies, and EGFR-TKIs are poorly effective against SqCC with EGFR mutations.6 However, SqCC with EGFR mutations have been found more frequently in never-smoker cases7; in addition, considering the present case and the previous report,2 osimertinib might show efficacy against EGFR-positive SqCC after the acquisition of T790M resistance mutation. To use EGFR-TKIs as a treatment alternative for EGFR-positive SqCC, EGFR mutations in SqCC should be examined.
T790M-positivity of the present case was diagnosed by liquid biopsy examination, and a histopathological examination of the re-biopsy specimen was not performed. Therefore, the transformation from SqCC to other histopathological subtypes cannot be denied. However, the tumour markers in the present case showed that the tumour was consistently CYFRA 21-1, and the other tumour markers were not elevated. These findings revealed that in the present case, the histopathological subtype probably did not transform to other subtypes during treatment.
Afatinib therapy has been described as a better treatment alternative for EGFR mutation-positive SqCC compared to erlotinib therapy. In LUX-Lung 8, a randomised phase III study, afatinib showed better progression free survival than erlotinib did in SqCC cases.8 Moreover, in a sub-analysis, afatinib showed further better progression-free survival than erlotinib did in ERBB (EGFR, HER2, HER3, and HER4) mutation-positive SqCC cases.9 In the present case, erlotinib was used as a first-line therapy because of the patient's low physical activity. However, afatinib should be used for EGFR mutation-positive SqCC, if possible. Further, in the future, it is important to evaluate which among the two EGFR-TKIs, afatinib or osimertinib, is more potent as the first-line treatment for EGFR mutation-positive SqCC.