Weaning from mechanical ventilation is one of the greatest volume and strength issues in evidence-based medicine in critically ill adults. In these patients, weaning protocols and daily interruption of sedation have been implemented, reducing the duration of mechanical ventilation and associated morbidity. In pediatrics, the information reported is less consistent, so that as yet there are no reliable criteria for weaning and extubation in this patient group. Several indices have been developed to predict the outcome of weaning. However, these have failed to replace clinical judgment, although some additional measurements could facilitate this decision.
La retirada de la ventilación mecánica es una de las temáticas con mayor volumen y solidez en medicina basada en la evidencia en adultos gravemente enfermos. La protocolización del destete y la interrupción diaria de la sedación han sido instauradas, reduciendo la duración de la ventilación mecánica y la morbilidad asociada en esta población. En pediatría la información reportada es menos consistente, propiciando que el destete y la extubación no cuenten aún con criterios de inicio objetivos y reproducibles. Diversos índices han sido desarrollados para predecir el resultado del destete; sin embargo, estos no han logrado reemplazar el juicio clínico, aunque algunas mediciones complementarias pudieran facilitar esta decisión.
Mechanical ventilation (MV) is a life-support therapy aimed at maintaining adequate alveolar ventilation and effective gas exchange in critically ill patients. The percentage of pediatric patients requiring MV and hospitalized in intensive care units (ICU) varies between 30% and 64%.1 While MV improves survival in these patients, it can lead to complications such as lung damage,2 MV-associated pneumonia,3 and dysfunction of the right ventricle.4 Therefore, weaning should be carried out as soon as the patient is able to maintain spontaneous breathing.
Ventilator disconnection, in a broad sense, includes two completely different but related situations: the progressive decline in ventilation (weaning) and the removal of the endotracheal tube (extubation).
Weaning can be defined as the gradual reduction in respiratory support, assigning a spontaneous breathing time to let the patient take responsibility for an acceptable gas exchange.5 This process can take between 40% and 50% of the total period receiving MV. However, some patients fail, prolonging the time with the ventilator. Various pathophysiological conditions have been linked to this failure, such as ventilatory overload, hemodynamic dysfunction, neuromuscular incompetence (central and/or peripheral), diaphragmatic muscle weakness, nutritional disorders, and metabolic disorders, among others.6 However, identifying the predominant mechanism remains a challenge for the treating team, as this is usually complex and multifactorial.
Extubation is the removal of the endotracheal tube. Generally, this point coincides with the determination that the patient is able to maintain an effective gas exchange without ventilator support or with minimal additional support. However, extubation has specific predictors of success and/or failure, which are usually associated with the ability to protect the airway, the management of secretions and patency of the upper respiratory tract.7
The term extubation failure (EF) represents a set of conditions that determine the need for reintubation and MV restoration within the first 24–72h after the removal of the endotracheal tube.5–8 About 55 studies in adults (approximately 33 000 patients) have reported an average EF rate of 12.5% (range 2%–25%). In pediatrics, the EF rate is equally heterogeneous, and varies between 4.9% and 29%.9 There is a controversy on the optimal EF rate, since very low values may reflect an unnecessary prolongation of MV, which would lead to an increased risk of MV-associated pneumonia, extended hospital stay and increased mortality. In this regard, Kurachek et al.7 reported that 62.5% of 136 unplanned extubations did not require reintubation, and so many of these children could have been extubated earlier than planned. In contrast, very high values would indicate early extubation, which is associated with potential catastrophic morbidities, primarily of a hemodynamic and respiratory nature.10 However, both situations may increase the duration of mechanical ventilation, ICU and hospital stays and, therefore, health care costs.11–13 Thus, the decision to initiate the weaning process and perform extubation should be based on objective and reproducible criteria, which in pediatrics still has a limited level of evidence.
The objective of this review was to analyze the available information on weaning and extubation in children, comparing it with the most important studies on the subject in the adult population. Based on current information, we suggest ways to address this decision in seriously ill children.
Weaning ProtocolsThe decision to start weaning depends on the fulfillment of certain clinical criteria, such as control of what caused the connection to MV, the effective gas exchange, an appropriate neuromuscular condition, an appropriate level of consciousness allowing the protection of airways, and a stable hemodynamic status.14 This decision usually lies with the intensive care physician, who begins the process when a possible successful weaning is suspected.6 However, application of weaning protocols guided both by nurses and respiratory therapists suggests that early identification of patients able to conduct a spontaneous breathing trial (SBT) through the evaluation of specific clinical criteria, reduces weaning time, duration of mechanical ventilation and ICU stay in adult patients.15–21 One of the first reports on the subject, by Ely et al.,21 showed that a daily assessment of certain criteria (PaO2/FiO2≥200, positive end-expiratory pressure (PEEP)≤5cmH2O, presence of cough reflex, respiratory rate/tidal volume ratio (RR/VT)≤105, without vasopressors and sedatives) while applying SBT reduced the duration and complications associated with MV, lowering health care costs.
In pediatrics, the efficacy of weaning protocols is still controversial.22–24 Schultz et al.22 reported that the use of a protocol reduced the time devoted to weaning, compared to an intervention guided by the intensive care physician. However, both duration of MV and extubation time did not differ between groups. A multicenter prospective study carried out by the PALISI research group showed that the use of a weaning protocol in pediatric patients receiving MV who had experienced a prior weaning failure did not significantly reduce weaning time or EF rate compared with the standard procedure. In addition, the authors cautioned that overtreatment with sedatives delayed weaning time. This study suggests that assessment of specific clinical criteria, combined with daily interruption of sedation, could be effective in reducing the duration of mechanical ventilation in pediatric patients.24
In this regard, excessive sedation during MV is a major problem, extending ventilator stay. In adults, Kress et al.25 observed that daily interruption of sedation reduced the duration of mechanical ventilation and ICU stay and, additionally, the rate of adverse events did not increase, and better neurological evaluation was possible. In pediatrics, Jin et al.26 reported that implementation of a sedation protocol including the COMFORT scale reduced duration of MV, ICU stay, total dose of sedatives, and incidence of withdrawal symptoms. Recently, Foronda et al.27 implemented a daily assessment strategy coupled with SBT in 294 children receiving MV for more than 24h. They managed to reduce the duration of mechanical ventilation without increasing EF rate. According to the authors, the differences with the results obtained by Randolph et al.24 were associated with patient selection, since only patients who had experienced a previous weaning failure were included in the latter study. Moreover, it should be noted that, although the breathing parameters set before SBT application were relatively high in the study by Foronda et al.,27 this did not represent a risk factor for EF, so the authors speculated that the differences between the two research groups could be attributed to daily assessment with conservative criteria and reluctance to perform SBT in patients with high ventilation assistance, as occurred in the control group.
In this regard, we believe that daily assessment with integrated clinical and functional parameters might shorten the identification of patients eligible for SBT.28 Despite the disparity in the criteria used by different authors, in our unit we use the criteria described in Table 1.
Clinical Criteria to Start Weaning in Pediatric Patients Undergoing Mechanical Ventilation.
| 1. | Resolution or improvement of the cause of respiratory failure |
| 2. | Hemodynamic stability: absence or progressive decrease of vasoactive drugs |
| 3. | Adequate level of consciousness (COMFORT≥18) |
| 4. | Spontaneous respiratory effort |
| 5. | Discontinue sedatives |
| 6. | Discontinue muscle relaxants at least 24h |
| 7. | No clinical signs of sepsis |
| 8. | Cough reflex present |
| 9. | Correction of significant metabolic and electrolyte imbalances |
| 10. | Adequate gas exchange with PEEP≤8cmH2O and FiO2≤0.5 |
The weaning method most commonly used in pediatrics is the gradual reduction of ventilator settings during synchronized intermittent mandatory ventilation (SIMV).1 With this practice, MV removal is carried out when low respiratory rates are achieved. In addition, this mode is typically programmed with pressure support, which guarantees a specific tidal volume according to patient needs, and would potentially have the advantage of reducing the additional respiratory effort imposed by the endotracheal tube and the mechanical circuit of the ventilator.5,29 However, this method in adults has been shown to extend MV, compared to the use of daily SBT and pressure support.30,31 Esteban et al.30 reported that weaning time depends on the technique used, where daily SBT reduces weaning time compared to SIMV and pressure support. Correspondingly, two studies showed that not all children need this gradual reduction to achieve a successful weaning.22,29
Spontaneous Breathing Trial MethodsSBT is performed while the patient is intubated and evaluates his/her cardiorespiratory tolerance to maintain spontaneous breathing without or with minimum respiratory support, thus allowing the identification of patients eligible for extubation. During this test, variables associated with respiratory muscle fatigue and the achievement of an effective gas exchange are measured.27,29,31 Several reports in adult patients have shown that between 60% and 80% of patients under MV can be smoothly extubated after successful tolerance of SBT.13,30–33
The most used SBT methods are continuous positive pressure airway (CPAP), T-tube and pressure support. In pediatrics, the choice will depend largely on the experience of the treating team, since none of these methods has been shown to be superior to the others.29,34 However, a recent study suggests that the use of high pressure, programmed according to the endotracheal tube diameter, might overestimate the success of SBT in pediatric patients, contributing to an increase in EF rate.35
Jones et al.33 compared CPAP and T-tube techniques for 60min in 106 adult patients, finding no difference in EF rate. In agreement with this, Esteban et al.13 found no difference in the use of T-tube or 7-cmH2O pressure support for 120min. These results were recently further corroborated by Molina-Saldarriaga et al.36 in patients with chronic obstructive pulmonary disease.
Farias et al.29 replicated these studies in 257 pediatric patients under MV for at least 48h, and found no difference between T-tube and 10-cmH2O pressure support in SBT tolerance or EF rate. Subsequently, the same authors assessed 418 pediatric patients with a similar method, noting that respiratory rate (RR), tidal volume (VT), weight-corrected RR and VT ratio (FR/VT), and maximal inspiratory pressure (MIP) measured during SBT were poor predictors of EF.37
The duration of SBT is also controversial. Most studies have established a duration of 120min.13,29,30,32 However, a prospective, randomized, multicenter study of 526 adult patients showed that a 30-min SBT identifies patients eligible for extubation with results equivalent to those obtained with 120-min SBT, with no difference in EF rate.31 Moreover, Chavez et al.38 used a novel form of SBT, with an adapted continuous-flow anesthesia bag (3 l/min for infants and 10 l/min for older children) adjusted to give 5cmH2O CPAP for 15min. They found that, of 70 pediatric patients, 7.8% experienced EF. However, it should be noted that those patients subjected to SBT were to be extubated according to the criteria of the intensive care physician in charge, so SBT might have been unnecessary.
Predictors of Extubation FailureOver 50 weaning predictors have been studied in adults. However, only five have shown a modest ability to predict weaning from MV (MIP, respiratory minute volume [MV], RR, VT, RR/VT).10 In this regard, measurement of indices composed of more than one variable, such as RR/VT and CROP index (compliance, resistance, oxygenation, pressure) have shown to be more accurate compared to MIP and MV, these having moderate predictive ability in adults.39 However, pediatric studies have reported that both RR/VT and CROP index have low predictive value,8,40,41 and only one study has shown potential usefulness of CROP index in predicting the outcome of extubation.42 This discrepancy could be attributed to the wide range of age and weight of pediatric patients, and to the disparity in the use of these predictors in pediatric practice compared to that in adult population.37,39
Thiagarajan et al.43 showed that RR≤45 breaths/min (bpm), VT≥5.5ml/kg, weight-adjusted RR/VT≤8bpm/ml/kg, and CROP index≥0.15ml/kg/min were cutoff values that predicted successful extubation. On the other hand, Baumeister et al.42 used RR/VT and CROP index to predict successful extubation, showing cutoff values different from those reported by Thiagarajan et al.43 (RR/VT≤11bpm/ml/kg and CROP≥0.1ml/kg/min). Conversely, Manczur et al.41 reported that measurements of VT and MV before extubation were more sensitive and specific with respect to multivariate measurements to determine the prognosis of extubation in pediatric patients. Venkataraman et al.44 showed that the analysis of variables such as VT, peak inspiratory pressure, dynamic compliance, and the ratio between VT and inspiratory time prior to extubation were able to predict EF in 312 pediatric patients under MV for more than 24h. Consistent with Manczur et al.,41 these authors observed that both RR/VT and CROP index were not useful for predicting the outcome of extubation.
Furthermore, the ability to maintain spontaneous breathing during weaning has been shown to depend on the central respiratory control, the capacity of the inspiratory muscles and the load placed on them.45–49 Manczur et al.45 observed that negative pressure measured at 0.1s after airway occlusion pressure (P0.1) was lower in pediatric patients who experienced EF. This result was attributed to poor central respiratory control, decreased inspiratory muscle strength and hyperinflation.
Likewise, studies in adults have shown that measurement of tension-time index (TTI) and the load/capacity balance, which are related to resistance to fatigue of respiratory muscles, optimize precision in the weaning prognosis.47,48 However, studies in children have shown conflicting results regarding the usefulness of these indices.46,49,50 Harikumar et al.46 reported that TTI measurement achieved a sensitivity and specificity of 100% for predicting EF, with a cutoff of 0.18. In contrast, Noizet et al.49 found that TTI, including multivariate indices, was unable to predict EF, concluding that the main cause of inefficiency of these indices is related to the time of measurement, as these parameters are measured when the patient already meets criteria for successful weaning. Recently, Johnston et al.50 reported that the measurement of VT, MV, MIP and load/capacity balance allowed for EF prediction in patients with severe acute bronchiolitis. The values of load/capacity balance were significantly lower in the successfully extubated group compared to the group that had EF, although the predictive ability of this index was low.
In this context, univariate indices seem to be a better alternative than integrated indices for predicting EF. However, cut-off values encompassing the entire pediatric population are still to be established, as well as the determination of the most appropriate time point to perform these measurements. Predictive indices according to extubation outcome and their cut-off values in pediatric patients are summarized in Table 2.
Predictors of Extubation Outcome and Cutoff Values in Pediatric Patients.
| Reference | First author (year) | Prediction | Indexes |
| 42 | Baumeister (1997) | Success | RR/VT<11bpm/ml/kgCROP≥0.1ml/kg/bpm |
| 8 | Farías (1998) | Failure | RR/VT>11bpm/ml/kgVT≤4ml/kg |
| 43 | Thiagarajan (1999) | Success | RR≤45bpmVT≥5.5ml/kgRR/VT≤8bpm/ml/kgCROP≥0.15ml/kg/bpm |
| 41 | Manczur (2000) | Failure | VT<6ml/kgMV<180ml/kg |
| 44 | Venkataraman (2000) | Failure | VT≤3.5ml/kg |
| 37 | Farías (2002) | Failure | RR≥45bpmVT≤4ml/kgRR/VT≥11bpm/ml/kgMIP≤20cmH2OPaO2/FiO2≤200 |
| 46 | Harikumar (2009) | Failure | MIP≤32.6cmH2OP0.1>4.45cmH2O |
| 50 | Johnston (2010) | Failure | RR/VT≥6.7bpm/ml/kgMIP≤50cmH2OMV<0.8ml/kg/min |
CROP: compliance, resistance, oxygenation, pressure index; FiO2: fraction of inspired oxygen; RR: respiratory rate; PaO2: partial pressure of oxygen; MIP: maximal inspiratory pressure; P0.1: airway occlusion pressure at 0.1s; bpm: breaths per minute; MV: minute volume; VT: tidal volume.
In adults, EF has been associated with increased duration of mechanical ventilation, ICU stay, health care costs, and mortality, so prevention is of vital importance. However, no parameters enabling accurate EF prevention are available to date, so being alert to the risk factors associated with EF is mandatory, in order to optimize the decision to extubate a patient receiving MV.51
Obstruction of the upper airway after extubation is among the most frequent causes of EF.7,50–52 Its prediction is complex, since SBT only investigates cardiorespiratory tolerance to spontaneous breathing. Wratney et al.52 reported that a cuff-leak test before endotracheal tube removal should not be used as the sole criterion for deciding extubation of pediatric patients, since the absence of leakage after application of air pressure equal to or greater than 30cmH2O (negative test) was common in this population, and no association was observed with the presence of stridor and/or need for reintubation. However, when facing the possibility of EF due to upper airway obstruction, prophylactic administration of corticosteroids has been shown to reduce the incidence of post-extubation stridor in the neonatal and pediatric population.53
Studies in children have shown conflicting results regarding EF rate and its associated risk factors.7,24,54–57 This has been partly attributed to the study design (retrospective vs prospective), inclusion criteria, and the number of participating centers5 (Table 3). In a retrospective study, Baisch et al.54 observed an EF rate of 4.1% in 3193 pediatric patients weaned from MV. The causes of EF were multifactorial, with obstruction of the upper airway being the main cause. Risk factors associated with EF were young age, longer MV duration, and longer ICU and hospital stay, but this was not associated with hospital mortality risk. Meanwhile, Edmunds et al.55 showed an EF rate of 7.9% in 280 patients intubated for at least 48h. Upper airway obstruction was again the most frequent cause of EF, and an association between MV duration and EF was also established. Fontela et al.56 reported an EF rate of 10.5%, observing that the main cause was respiratory failure. In this study, risk factors associated with EF were young age, MV duration>15 days, oxygenation index>5, use of inotropes and intravenous administration of sedative drugs>10 days. Kurachek et al.,7 in a prospective multicenter study involving 16 ICUs, reported an EF rate of 6.2% (range, 1.5%–8.8%) in 1459 pediatric patients intubated for at least 24h, with an average MV duration of 4.8 days. The risk factors for EF were age younger than 24 months, genetic syndrome, and chronic respiratory and neurological impairment, in addition to the need for endotracheal tube replacement at admission. The incidence of upper airway obstruction after extubation was 37%, and the authors found no association between MV duration and EF rate. However, a more rigorous analysis including only patients who stayed over 48h in MV revealed an increased EF rate (from 4.2% to 8%). Our experience agrees with the above mentioned studies, since we observed an EF rate of 5.3% directly associated with MV duration and the duration of continuous administration of opiates and benzodiacepines.57
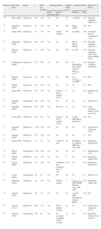
Analysis of Different Items Related to Weaning From Mechanical Ventilation in Pediatrics.
| Reference | First author (year) | Design | n | Mean age (months) | Weaning protocol | Duration of MV (days) | Extubation failure | Main cause of EF (%) | ||
| Daily assessment | Type of SBT | Predictors | Rate | |||||||
| 40 | Khan (1996) | Prospective | 208 | 39.4 | No | No | 5.1 | VT, RR/VT | 16.3 | Increased respiratory effort (41.2) |
| 42 | Baumeister (1997) | Prospective | 47 | 36.5 | No | No | ND | RR/VT, CROP | 19.1 | ND |
| 8 | Farías (1998) | Prospective | 84 | 7.5a | No | T-tube, 120min | 8.5a | VT, RR/VT | 16.0 | Decreased level of consciousness (33.3) |
| 43 | Thiagarajan (1999) | Prospective | 227 | 49.9 | No | No | 6.1 | RR, VT, RR/VT, CROP | 11.0 | Increased respiratory effort (82.1) |
| 41 | Manczur (2000) | Prospective | 47 | 46.8 | No | No | ND | VT, MV | 14.9 | Increased respiratory effort (42.9), hypoventilation (42.9) |
| 44 | Venkataraman (2000) | Prospective | 312 | ND | No | No | No | VT, preextubation FiO2, MAP, OI, FrVe, PIP, Edin, VT/Ti | 16.0 | Increased respiratory effort (40.0) |
| 45 | Manczur (2000) | Prospective | 42 | 14.4 | No | No | ND | P0.1, P0.1/P0.1 max, P0.1/MIP | 14.3 | ND |
| 22 | Schultz (2001) | Prospective | 219 | 55.4 | No | No | 3.4 | No | 2.7 | ND |
| 29 | Farias (2001) | Prospective | 257 | 11.0 | No | T-tube or PS 10cmH2O. 120m | 6 | No | 13.9 | Multifactorial (82.1) |
| 55 | Edmunds (2001) | Retrospective | 548 | 52.4 | No | No | 6.5 | No | 4.9 | Stridor (22.2) |
| 24 | Randolph (2002) | Prospective | 182 | ND | Yes | PS 120m | 8.7 | No | 18.4 | Acute respiratory failure of lower tract (54.2) |
| 37 | Farias (2002) | Prospective | 418 | 9.9 | No | T-tube or PS 10cmH2O 120m | 6.6 | VT, RR, MIP, RR/VT, PaO2/FiO2 | 14.0 | ND |
| 7 | Kurachek (2003) | Prospective | 2.794 | 15.5a | No | No | 4.6 | No | 6.2 | Upper airway obstruction (37.3) |
| 23 | Restrepo (2004) | Prospective | 187 | 32.8 | No | No | 2.1 | No | 4.3 | ND |
| 49 | Noizet (2005) | Prospective | 57 | 28 | No | T-tube. 30min | 4.4 | RR, RR/VT, P0.1×RR/VT, MIP, PTI, TTI1, TTI2 | 21.1 | Respiratory failure (50.0) |
| 54 | Baisch (2005) | Retrospective | 3.193 | 55.0 | No | No | 5.2 | No | 4.1 | Multifactorial (32.3) |
| 56 | Fontela (2005) | Prospective | 124 | 19.5 | No | No | 6 | No | 10.5 | Respiratory failure (76.9) |
| 38 | Chavez (2006) | Prospective | 70 | 16.5a | No | Continuous flow anesthesia bag. 15min | 3.27a | No | 11.0 | Respiratory failure (45.5) |
| 57 | Cruces (2008) | Retrospective | 151 | 9.7 | No | No | 4.2 | No | 5.3 | Stridor (37.5) |
| 46 | Harikumar (2009) | Prospective | 80 | 25.2 | No | CPAP 5cmH2O | 3 | TI/TTOT, P0.1, MIP, IP/MIP, FRC/kg, TTmus, PIP, FiO2 | 10.0 | Respiratory failure (100) |
| 50 | Johnston (2010) | Prospective | 40 | 4.2 | No | No | 6.3 | VT, MV, MIP, load/capacity balance, RR/VT | 15.0 | ND |
| 27 | Foronda (2011) | Prospective | 260 | 11.3 | Yes | PS 10cmH2O. 120min | 4.1 | No | 12.7 | Upper airway obstruction (57.5) |
| 35 | Ferguson (2011) | Retrospective | 538 | 48 | Yes | PS according to diameter of ETT, 120min | 1.5 | No | 11.2 | Respiratory failure of lower tract (54) |
CPAP: continuous positive airway pressure; FRC/kg: functional residual capacity indexed to patient weight; CROP: compliance, resistance, oxygenation, pressure index; Edin: dynamic elasticity; SBT: spontaneous breathing trial; EF: extubation failure; FiO2: fraction of inspired oxygen; RR: respiratory rate; FrVe: fraction of the total minute ventilation provided by the ventilator; OI: oxygenation index; PTI: pressure time index; ND: no data; PaO2/FiO2: ratio of arterial oxygen pressure/fraction of inspired oxygen; MIP: maximal inspiratory pressure; IP/MIP: ratio of inspiratory airway pressure and maximal inspiratory pressure; PIP: peak inspiratory pressure; MAP: mean airway pressure; P0.1: airway occlusion negative pressure at 0.1s; P0.1max: maximal airway occlusion negative pressure at 0.1s; PS: pressure support; ETT: endotracheal tube; TTI1: tension-time index obtained from P0.1; TTI2: tension-time index obtained from MAP; TI/TTOT: ratio of inspiratory time/total time of respiratory cycle; TTmus: tension-time index of the inspiratory muscles; MV: mechanical ventilation; MV: minute volume; VT: tidal volume; VT/Ti: average inspiratory flow.
Three common indications for noninvasive ventilation (NIV) during weaning have been described in adults: (a) as an alternative weaning mode for patients presenting with SBT failure; (b) as prophylaxis after extubation in patients without respiratory failure at high risk of reintubation; and (c) as support in extubated patients with respiratory failure within the first 48h after extubation. The first strategy is aimed at facilitating weaning, and the last two at trying to avoid reintubation.6,58 However, studies on the topic have shown conflicting results regarding the usefulness of NIV during weaning.58–62
A meta-analysis by Burns et al.59 evaluated the use of NIV as an alternate modality to help early weaning, observing that this strategy was associated with a significant reduction in mortality, incidence of pneumonia and MV duration. Furthermore, the subgroup analysis revealed that the benefits of NIV in relation to mortality were higher in patients with chronic obstructive pulmonary disease.
Meanwhile, Ferrer et al.60 showed that NIV used as rescue therapy immediately after extubation reduced the risk of acute respiratory failure in patients with chronic hypercapnic respiratory failure during SBT, even reducing mortality at 90 days. In contrast, two studies reported no difference in the reintubation rate when comparing the use of NIV and standard therapy in patients with respiratory failure after extubation.61,62 Additionally, Esteban et al.62 reported higher mortality in the group of patients subjected to NIV, probably associated with delayed reintubation.
In pediatrics, the experience with NIV during weaning is limited, because the available information is from uncontrolled studies and case series with few patients.63 Mayordomo-Colunga et al.64 reported that NIV was more effective in preventing reintubation when used early in patients at high risk of EF, compared to its use as rescue therapy in patients with established respiratory failure. In addition, the authors observed that the reduction in RR and FiO2 after 6h was associated with the success of NIV. Likewise, James et al.65 agreed that both measurements, combined with pH and the underlying pathology, are criteria to consider when predicting the effectiveness of NIV in pediatric patients.
In another interesting aspect of NIV, Fauroux et al.66 recently reported beneficial effects during decannulation in a group of select pediatric patients with severe upper airway obstruction, as well as in the treatment of respiratory failure after decannulation.
Nevertheless, integration of NIV in weaning requires preset criteria for initiation and failure, which are not yet fully defined and validated in pediatrics. In the future, the transitional use of NIV in weaning from MV could be considered successful if it facilitates weaning and/or prevents reintubation.67
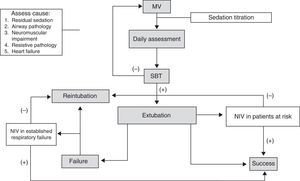
ConclusionsBased on the information discussed here, we believe that the daily assessment of specific clinical and functional criteria combined with SBT allows for early identification of patients eligible for weaning from MV. Daily suspension of sedation shortens the duration of MV, and assessment using the COMFORT scale prevents excessive sedation in pediatric patients. Univariate predictors before weaning appear to be more effective in determining prognosis of extubation. Not all pediatric patients require a protocolized weaning strategy. Taking these measures may be more effective in pediatric patients with risk factors such as young age, longer duration of MV, genetic syndromes, and chronic respiratory and neurological disorders, who are usually excluded from weaning studies in pediatric patients. Despite the high incidence of EF from upper airway obstruction, absence of audible leak around the endotracheal tube with an airway pressure higher than 30cmH2O should not delay the decision to extubate, since prophylactic administration of corticosteroids has shown positive results, reducing this condition. We need more studies to recommend routine use of NIV after extubation, although some promising indications in select groups of pediatric patients have been reported. In patients with established respiratory failure requiring NIV, clinical response should be determined, considering the increased mortality due to delayed reintubation. Our proposed algorithm for MV withdrawal in pediatric patients is detailed in Fig. 1. Finally, we believe that further studies comparing weaning methods and protocols in the most complex patients are needed, since they will probably benefit the most from a suitable choice of weaning strategy.
FundingThe study has not received any funding.
Conflicts of InterestThe authors declare no potential conflicts of interest.
The authors gratefully acknowledge the contribution of physiotherapists Ramón Pinochet and Máximo Escobar in careful review of the manuscript.
Please cite this article as: Valenzuela J, Araneda P, Cruces P. Retirada de la ventilación mecánica en pediatría. Estado de la situación. Arch Bronconeumol. 2014;50:105–112.