Recent studies have found variability in asthma inflammatory phenotypes determined by the inflammatory cells in induced sputum (IS). The aim of this study was to determine the frequency and factors affecting inflammatory phenotype variability in IS.
MethodsRetrospective observational study that included 61 asthmatic patients who underwent at least two IS tests over a period of 5 years. They were classified according to their baseline inflammatory phenotype and subsequently grouped according to phenotype variability (persistent eosinophilic, persistent non-eosinophilic and intermittent eosinophilic). Demographic, clinical and functional data and factors potentially influencing IS variability were collected in all cases.
ResultsOf the 61 patients, 31 (50.8%) had a change with respect to baseline inflammatory phenotype. Of these, 16 (51.6%) were eosinophilic, 5 (16.1%) neutrophilic, 1 (3.2%) mixed and 9 (29.1%) paucigranulocytic. According to phenotype variability, 18 patients (29.5%) were classified as persistent eosinophilic, 17 (27.9%) non-persistent eosinophilic, and 26 (42.6%) intermittent eosinophilic. Smoking and recent asthma exacerbation were significantly associated with increased risk of variability of the IS inflammatory phenotype (OR=6.44; P=.013; 95% CI=1.49–27.80 and OR=5.84; P=.022; 95% CI=1.29–26.37, respectively).
ConclusionHalf of asthma patients, predominantly those with eosinophilic phenotype, present a change in IS inflammatory phenotype. This variability is associated with smoking and recent asthma exacerbation. Data suggest these factors can modify the classification of IS inflammatory phenotype in clinical practice.
Estudios recientes han constatado variabilidad del fenotipo inflamatorio del asma en el recuento de las células inflamatorias del esputo inducido (EI). El objetivo del presente estudio fue determinar la frecuencia y los factores que condicionan la variabilidad del fenotipo inflamatorio del EI.
MétodosEstudio observacional retrospectivo que incluyó 61 pacientes asmáticos a los que se les practicó un mínimo de dos EI en un período de 5 años. Los pacientes fueron clasificados según su fenotipo inflamatorio y posteriormente agrupados según la variabilidad del fenotipo (eosinofílicos persistentes, no eosinofílicos persistentes y eosinofílicos intermitentes). De todos los casos incluidos se recogieron datos demográficos y clínico-funcionales, así mismo se valoró los factores que pudiesen influir en la variabilidad del EI.
ResultadosDe los 61 pacientes, 31 (50,8%) presentaron un cambio del fenotipo inflamatorio inicial. De estos, 16 (51,6%) eran eosinofílicos, 5 (16,1%) neutrofílicos; 1 (3,2%) mixto y 9 (29,1%) paucigranulocíticos. Según la variabilidad, 18 pacientes (29,5%) se clasificaron como eosinofílicos persistentes, 17 (27,9%) no eosinofílicos persistentes y 26 (42,6%) eosinofílicos intermitentes. El tabaquismo y una exacerbación asmática reciente se asociaron significativamente con mayor riesgo de variabilidad del fenotipo inflamatorio del EI (OR=6,44; p=0,013; IC95%=1,49–27,80 y OR=5,84; p=0,022; IC95%=1,29–26,37, respectivamente).
ConclusiónLa mitad de los pacientes asmáticos modifican el fenotipo inflamatorio del EI, predominando los de fenotipo eosinofílico. Esta variabilidad se asocia al tabaquismo y a una exacerbación asmática reciente. Los datos sugieren que estos factores podrían influir en la determinación del fenotipo inflamatorio del EI en la práctica clínica habitual.
The study of bronchial inflammation has become a highly useful tool for characterizing and monitoring patients. Non-invasive techniques, such as inflammatory cell counts in induced sputum (IS), have been developed and applied to great benefit in determining inflammatory phenotypes in severe asthma and tailoring patient treatment.1,2 Identification of the inflammatory phenotype from IS is more precise than determinations made from the fraction of exhaled nitric oxide (FENO).3 Various clinical practice guidelines recommend the use of IS in the clinical evaluation of patients with uncontrolled severe asthma.1,4
Four inflammatory phenotypes have been described in asthma patients, determined according to IS cellularity: eosinophilic, neutrophilic, mixed, and paucigranulocytic.5 The identification of these phenotypes is not merely of academic interest; it can be used to guide treatment. For example, patients with non-eosinophilic phenotypes benefit less from treatment with inhaled corticosteroids (IC) than eosinophilics,6,7 and it has been suggested that patients with neutrophilic phenotype respond favorably to long-term macrolide treatment,8 and possibly to tiotropium.9
Nevertheless, the reliability of cell counts in IS has been questioned following recent reports of changes in the proportion of inflammatory cells in IS samples obtained from the same patient at different times. This variability has been reported to occur over the course of the day,10 due to the effect of IC treatment,11,12 or over the course of the disease, both in adults13 and in children.14 Other studies, however, have not reported any variability.6,15 Be that as it may, the current recommendation is to perform more than one test in each patient in order to establish the correct inflammatory phenotype.14,16,17
Aside from the issues concerning the existence and incidence of this variability, little information is available on the factors associated with this phenomenon. An understanding of the causes of variability in inflammatory cell counts in IS would be of great importance in clinical practice. It would be useful, firstly, to identify the potential limitations of the IS technique for identifying asthma inflammatory patterns; and secondly, to anticipate or prevent such variability, if possible. The primary objectives of this study, then, were to determine the frequency of IS inflammatory phenotype variability in asthma and to identify factors that might cause phenotypes to change.
MethodsDesignThis was a retrospective, observational study, analyzing the IS of 61 asthma patients on maintenance treatment, aged between 18 and 85 years. Asthma diagnosis was established if patients had consistent symptoms and confirmed variable airflow limitation (positive bronchodilator test, diurnal variability in peak expiratory flow >20% over 2 weeks or positive methacholine bronchial challenge test), according to the criteria of the Spanish Guideline on the Management of Asthma (GEMA 2009).4 Study data were obtained from the clinical records of patients who attended asthma specialist clinics in our hospital and in whom IS testing had been performed either for clinical reasons (discrepancy between symptoms and FENO results, poor asthma control, and/or suspected concomitant gastroesophageal reflux) or due to their participation in another study (for determining inflammatory phenotype). All patients had undergone at least 2 IS tests between 2008 and 2013. The study design complied with the principles of the Declaration of Helsinki (1964) and was part of a subanalysis of another study approved by the Hospital Ethics Committee (ClinicalTrials.gov: NCT02028637).
ProceduresDemographic and clinical data were collected, including sex, age, smoking history, defined as active smokers or former smokers if they had stopped for at least 1 year. Asthma severity and stratification of IC doses were established according to GEMA 20094 and the Global Initiative for Asthma (GINA).18 The Asthma Control Test (ACT), a self-administered questionnaire validated in Spanish, was used to evaluate asthma control.19 Asthma exacerbation was any episode (infectious or otherwise) that changed the previous clinical status of the patient, irrespective of the severity of the event, according to ATS/ERS criteria.20 Change in corticosteroid dose was defined as any modification in the patient's treatment involving the introduction, increase, discontinuation or reduction of inhaled or systemic corticosteroid treatment. Clinical and other data were collected within a period of 3 months prior to IS sampling.
Study procedures: skin prick testing was performed for common airborne allergens.21 Spirometry was performed using a Datospir-600 (Sibelmed SA, Barcelona, Spain) device, according to SEPAR 2013 recommendations.22,23 FENO was performed using an electrochemical device (NO Vario Analyzer. FILT Lungen and Thorax Diagnostic GmbH, Berlin, Germany) at a flow of 50mL/s, according to ATS/ERS 2005 recommendations.24 FENO levels of ≥50ppb were considered significantly elevated.25 Total serum IgE was determined using ImmunoCAP (Phadia 250). Sputum samples were induced and processed according to the standardized procedure published by Pizzichini et al.: all patients were premedicated with 200μg of inhaled salbutamol. After 10min of bronchodilation, IS was obtained after inhalation of a hypertonic solution aerosol for 7min at each concentration (3%, 4% and 5%). Particles were generated in an ultrasonic nebulizer (Omron NE U07) with a mean diameter of 7μm and an output of 3ml/s. As a safety precaution, FEV1 was also measured after each inhalation period. Mucus plugs were treated with dithiothreitol (Sputolysin, Calbiochem Corp., San Diego, CA, USA) and phosphate-buffered saline solution and sputums were processed within 2h. The cell suspension was filtered, and the total number of cells per gram of sputum, viability and total squamous cells indicating upper respiratory tract contamination were calculated using a blood cell counter and Trypan blue staining. Cell preparation and supernatant were obtained by cytocentrifugation. May–Grünwald–Giemsa staining was applied to this preparation for the differential cell count.26
Inflammatory Phenotypes in AsthmaInflammatory phenotypes in IS were classified into 4 groups, as described in previous studies by Simpson et al.,5 Fleming et al.14 and Haldar and Pavord,16 as follows: eosinophilic (eosinophils>2.5%); neutrophilic (neutrophils>64%); mixed (eosinophils>2.5% and neutrophils>64%); and paucigranulocytic (eosinophils<2.5% and neutrophils<64%).
To define changes in the IS inflammatory phenotypes between the 2 samples, we used the classification previously described in similar studies, given the population difference between the 4 initial phenotypes.14,27 Patients were thus classified into 3 groups: persistent eosinophilic (eosinophils>2.5% in both IS), persistent non-eosinophilic (eosinophils<2.5% in both IS), and intermittent eosinophilic (eosinophils>2.5% in either IS). The characteristics of the 3 groups were compared to identify variables associated with the presence or absence of phenotype variability.
Statistical AnalysisResults were expressed as means and standard deviation for continuous variables with normal distribution. For continuous variables with non-normal distribution, values were expressed as median and range, and for categorical variables, frequency and percentages were used. An analysis of variance (ANOVA) was used to compare demographic and clinical data between the 3 groups. A multivariate analysis was performed with logistic regression to identify variables independently associated with changes in inflammatory phenotype in the second sputum test. We also included in this analysis variables with statistically significant differences between the 3 patient groups. Results were considered significant when P<.05. Missing data were not imputed. All analyses were performed using SPSS (V.18) and GraphPad Prism V5.
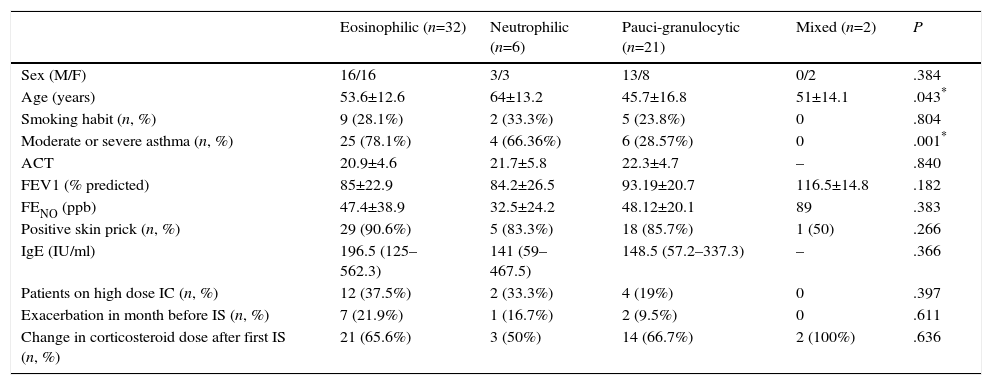
ResultsA total of 61 patients were included, of whom 32 (52.5%) were men; mean age was 51.8 years. Demographic and clinical data of the study population by initial inflammatory phenotype are listed in Table 1.
Demographic and Clinical Characteristics According to Initial Inflammatory Phenotype.
| Eosinophilic (n=32) | Neutrophilic (n=6) | Pauci-granulocytic (n=21) | Mixed (n=2) | P | |
|---|---|---|---|---|---|
| Sex (M/F) | 16/16 | 3/3 | 13/8 | 0/2 | .384 |
| Age (years) | 53.6±12.6 | 64±13.2 | 45.7±16.8 | 51±14.1 | .043* |
| Smoking habit (n, %) | 9 (28.1%) | 2 (33.3%) | 5 (23.8%) | 0 | .804 |
| Moderate or severe asthma (n, %) | 25 (78.1%) | 4 (66.36%) | 6 (28.57%) | 0 | .001* |
| ACT | 20.9±4.6 | 21.7±5.8 | 22.3±4.7 | – | .840 |
| FEV1 (% predicted) | 85±22.9 | 84.2±26.5 | 93.19±20.7 | 116.5±14.8 | .182 |
| FENO (ppb) | 47.4±38.9 | 32.5±24.2 | 48.12±20.1 | 89 | .383 |
| Positive skin prick (n, %) | 29 (90.6%) | 5 (83.3%) | 18 (85.7%) | 1 (50) | .266 |
| IgE (IU/ml) | 196.5 (125–562.3) | 141 (59–467.5) | 148.5 (57.2–337.3) | – | .366 |
| Patients on high dose IC (n, %) | 12 (37.5%) | 2 (33.3%) | 4 (19%) | 0 | .397 |
| Exacerbation in month before IS (n, %) | 7 (21.9%) | 1 (16.7%) | 2 (9.5%) | 0 | .611 |
| Change in corticosteroid dose after first IS (n, %) | 21 (65.6%) | 3 (50%) | 14 (66.7%) | 2 (100%) | .636 |
Values expressed as mean±standard deviation, median (range) or percentage, as indicated.
ACT, asthma control test; FENO, fraction of exhaled nitric oxide; FEV1, forced expired volume in 1s; IC, inhaled corticosteroid; IgE, immunoglobulin E; M/F, male/female; IS, induced sputum.
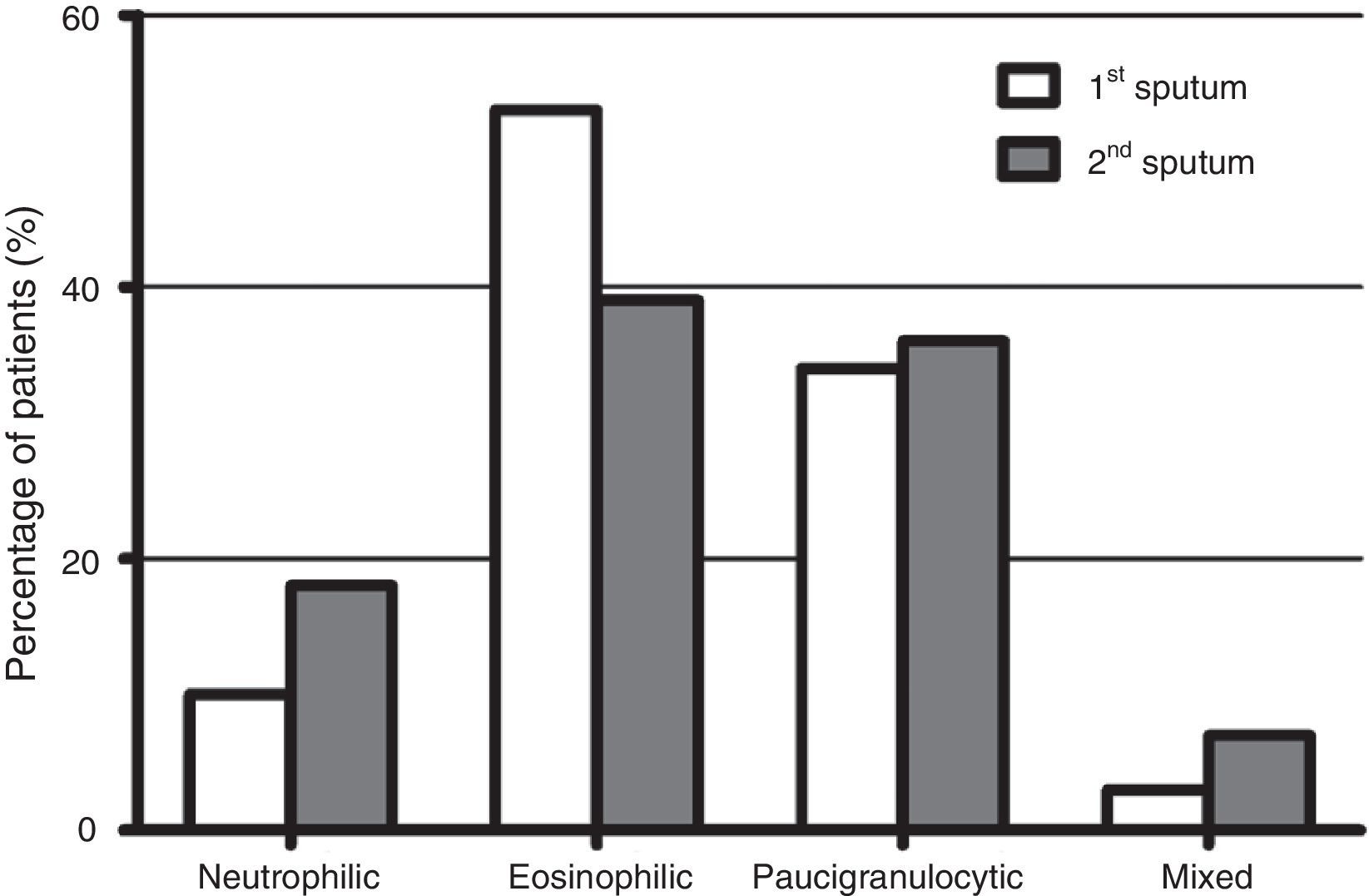
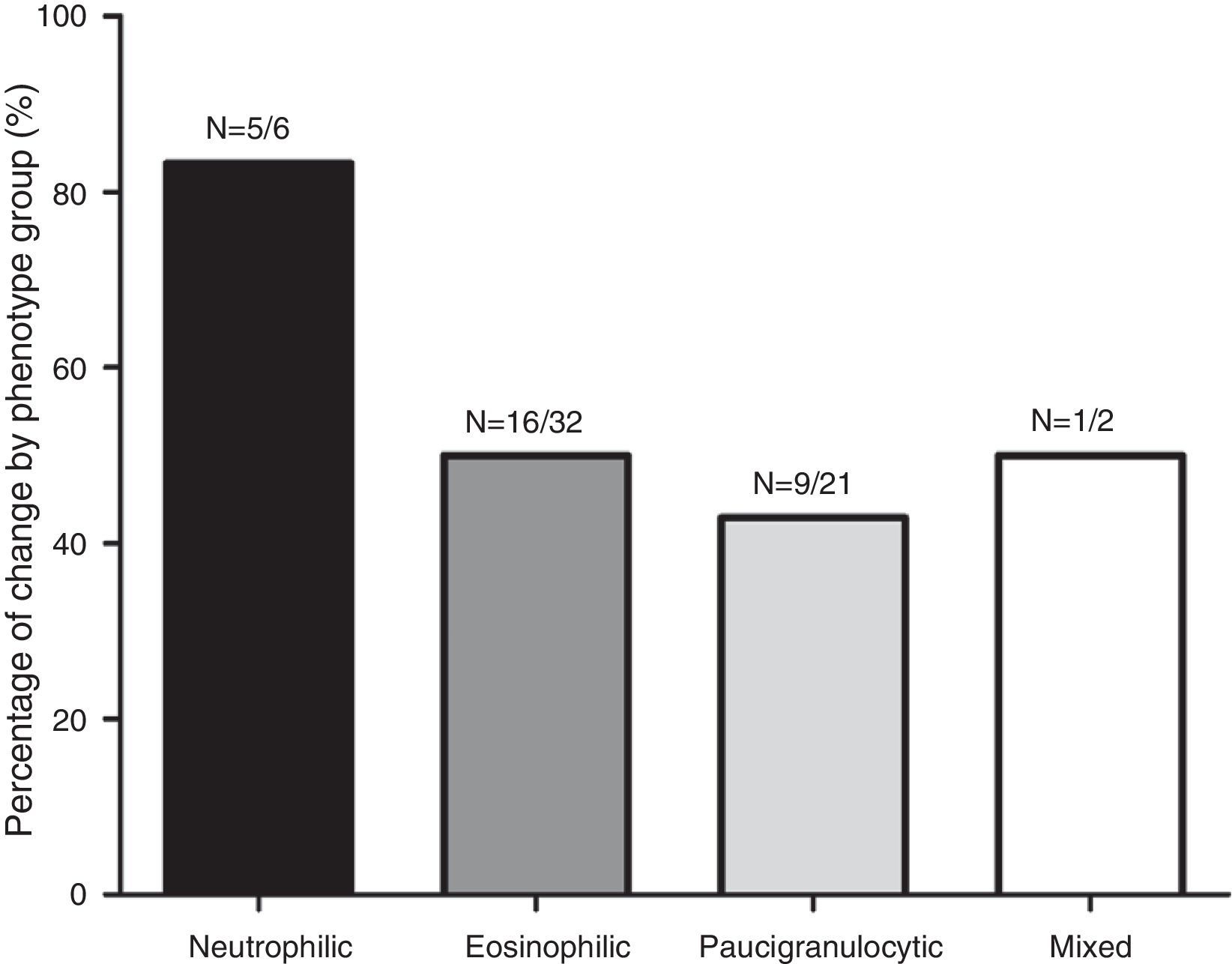
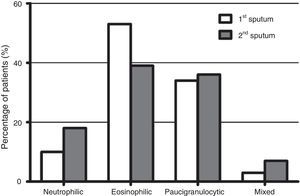
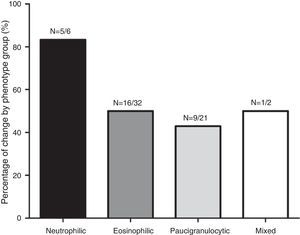
Fig. 1 shows the distribution of the inflammatory phenotypes observed in the first and the second induced sputum sample. The predominant phenotype in both instances was eosinophilic (52.5% and 39.3% respectively). In 31 patients, the phenotype changed in their second IS. Fig. 2 shows the percentage of patients whose inflammatory phenotype changed, by initial phenotype group.
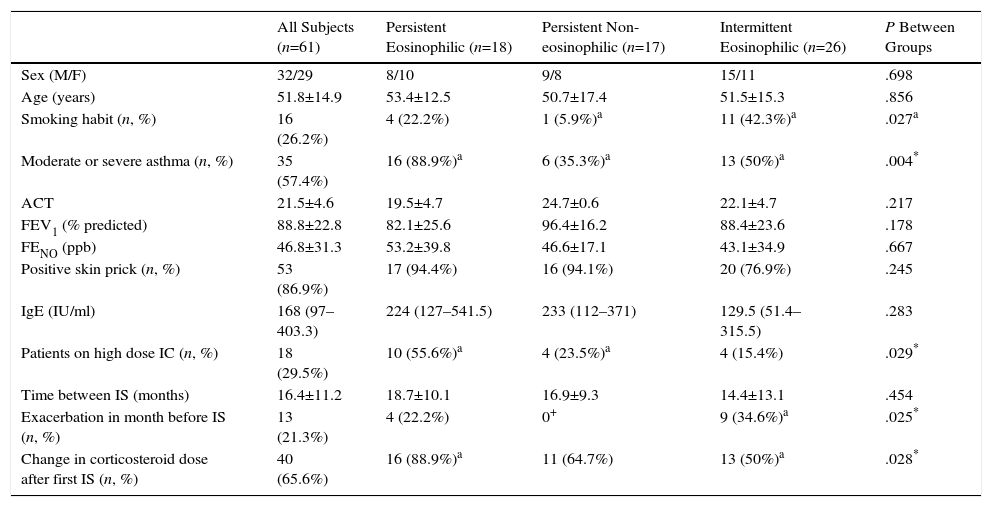
The analysis of inflammatory phenotype changes between IS sampling showed that 18 patients (29.5%) were persistent eosinophilic, 17 (27.9%) were persistent non-eosinophilic; and 26 (42.6%) were intermittent eosinophilic. Demographic and clinical features of the patients in these 3 groups are summarized in Table 2. In the intermittent eosinophilic group, we found a significantly higher rate of smokers and exacerbations in the previous month; in the persistent eosinophil group, there was a significantly higher proportion of patients with moderate or severe asthma who been receiving high dose ICs and had changed their corticosteroid dose after the first sputum test.
Demographic and Clinical Characteristics According to Change in Inflammatory Phenotype.
| All Subjects (n=61) | Persistent Eosinophilic (n=18) | Persistent Non-eosinophilic (n=17) | Intermittent Eosinophilic (n=26) | P Between Groups | |
|---|---|---|---|---|---|
| Sex (M/F) | 32/29 | 8/10 | 9/8 | 15/11 | .698 |
| Age (years) | 51.8±14.9 | 53.4±12.5 | 50.7±17.4 | 51.5±15.3 | .856 |
| Smoking habit (n, %) | 16 (26.2%) | 4 (22.2%) | 1 (5.9%)a | 11 (42.3%)a | .027a |
| Moderate or severe asthma (n, %) | 35 (57.4%) | 16 (88.9%)a | 6 (35.3%)a | 13 (50%)a | .004* |
| ACT | 21.5±4.6 | 19.5±4.7 | 24.7±0.6 | 22.1±4.7 | .217 |
| FEV1 (% predicted) | 88.8±22.8 | 82.1±25.6 | 96.4±16.2 | 88.4±23.6 | .178 |
| FENO (ppb) | 46.8±31.3 | 53.2±39.8 | 46.6±17.1 | 43.1±34.9 | .667 |
| Positive skin prick (n, %) | 53 (86.9%) | 17 (94.4%) | 16 (94.1%) | 20 (76.9%) | .245 |
| IgE (IU/ml) | 168 (97–403.3) | 224 (127–541.5) | 233 (112–371) | 129.5 (51.4–315.5) | .283 |
| Patients on high dose IC (n, %) | 18 (29.5%) | 10 (55.6%)a | 4 (23.5%)a | 4 (15.4%) | .029* |
| Time between IS (months) | 16.4±11.2 | 18.7±10.1 | 16.9±9.3 | 14.4±13.1 | .454 |
| Exacerbation in month before IS (n, %) | 13 (21.3%) | 4 (22.2%) | 0+ | 9 (34.6%)a | .025* |
| Change in corticosteroid dose after first IS (n, %) | 40 (65.6%) | 16 (88.9%)a | 11 (64.7%) | 13 (50%)a | .028* |
Values expressed as mean±standard deviation, median (range) or percentage, as indicated.
ACT, asthma control test; FENO, fraction of exhaled nitric oxide; FEV1, forced expired volume in 1s; IC, inhaled corticosteroid; IgE, immunoglobulin E; M/F, male/female; IS, induced sputum.
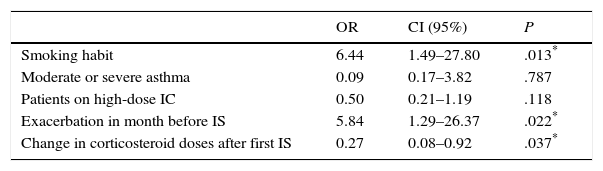
The logistic regression analysis used to identify causes independently associated with IS variability is shown in Table 3. In this table, it can be seen that patients with an asthma exacerbation in the month before the second IS (OR=5.84; P=.022; 95% CI=1.29–26.37) or a smoking habit (OR=6.44; P=.013; 95% CI=1.49–27.80) had a significantly higher risk of changing phenotype, while changes in corticosteroid doses between the two IS sampling times was a protective factor against change (OR=.27; P=.037; 95% CI=0.076–0.923). Moderate or severe asthma (OR=0.09; P=.787; 95% CI=0.170–3.824) and high IC doses (OR=0.50; P=.118; 95% CI=0.211–1.191) were not associated with changes in initial inflammatory phenotype.
Multivariate Analysis of Demographic and Clinical Characteristics Related with Change in IS Inflammatory Phenotype.
| OR | CI (95%) | P | |
|---|---|---|---|
| Smoking habit | 6.44 | 1.49–27.80 | .013* |
| Moderate or severe asthma | 0.09 | 0.17–3.82 | .787 |
| Patients on high-dose IC | 0.50 | 0.21–1.19 | .118 |
| Exacerbation in month before IS | 5.84 | 1.29–26.37 | .022* |
| Change in corticosteroid doses after first IS | 0.27 | 0.08–0.92 | .037* |
IC, inhaled corticosteroid; SI, induced sputum.
The main contribution of this study has been to confirm that smoking and recent asthma exacerbation constitute independent risk factors for changes in IS inflammatory phenotype in asthma patients. Moreover, our series confirms that up to half of cases undergo a change in inflammatory phenotype, and that the eosinophilic phenotype is the one that most often changes.
Current evidence of the stability of the inflammatory phenotype in asthma patients is contradictory and based on few studies. Two studies performed in asthma patients described stability in IS cell count in 2 determinations performed as far apart as 5 years.6,15 However, in other studies in which serial IS cell counts were performed over the course of a year in adults13,28 (monthly in patients with severe asthma and every 3 months in patients with moderate asthma) and during exacerbations,28 variability was observed in eosinophil and neutrophil concentrations in IS, particularly in patients with moderate and severe asthma; stability was observed in only one third of the series.13 Similarly, a recent study compared phenotypes of patients classified according to physiological variables or biomarkers, such as IS cellularity, over a period of 1 year. They found greater variability in IS variables, particularly in the more severe forms of the disease.29 In contrast, another study performed in children with different degrees of asthma control, found that changes in inflammatory phenotype were not associated with asthma severity.14 This evidence suggests that serial determinations of IS can improve the characterization of asthma in the individual throughout the course of the disease.29 However, the complexity of this disease means that in the future it may be also necessary to define patients by “endotype”, in addition to the clinical and inflammatory phenotypes currently in use.30
Our study showed that the initial inflammatory phenotype changed in half of the patients, with just over half of these changes occurring in the eosinophilic phenotype group. This variability was unrelated to sex, age, history of atopy, level of asthma control, serum IgE levels, FENO, or lung function values. These results differ from those of Schleich et al.31 who reported, in a study of 508 asthma patients, that high concentrations of eosinophils in blood, high FENO and IgE levels, and poor lung function test results were predictive of eosinophilia in IS.
Other factors, such as smoking, obesity, hormonal changes, respiratory infections, etc., are related to inflammatory cell concentrations in IS in asthma patients.2 We found a significantly greater proportion of smokers among the group with intermittent eosinophilia, probably because most of them initially had an eosinophilic phenotype, and because smoking was identified as a risk factor for inflammatory phenotype variability in logistic regression analysis. It is interesting to note that smoking is also associated with other more severe forms of asthma, a greater proportion of neutrophilic inflammation, and oxidative stress.32,33
Previous studies have shown that viral infections in asthmatic adults can trigger asthma or disease exacerbations.34,35 Neutrophilic inflammation in the bronchi caused by these infections is reflected in IS cellularity, and this may be a confounding factor when interpreting IS. Wark et al.34 studied IS in patients with an infectious (viral) and non-infectious exacerbation of their asthma. The first group showed predominantly neutrophilic phenotype, while the second group was eosinophilic. In our study, patients with an asthma exacerbation in the month before their second IS determination showed greater variability in eosinophil and neutrophil counts, suggesting that this is a risk factor for change in inflammatory phenotype. This provides a solid argument against performing IS inflammatory cell counts in patients with a recent exacerbation.
A positive correlation has been observed between a reduction in eosinophils in IS and improved FEV1 48h after starting treatment with IC.36 Accordingly, it has been suggested that monitoring eosinophil levels in IS may be useful for identifying the minimum dose of IC necessary to achieve control of bronchial inflammation.37 In our study, patients who changed their dose of corticosteroids had less variability in their initial inflammatory phenotype. This contradictory finding may be due in part to our relatively high proportion of patients with persistent non-eosinophilic asthma (27.9%), who are known to respond less to conventional asthma treatments than patients with eosinophilic phenotypes.38
It is well established that corticosteroid treatment causes eosinophil counts in IS to fall.39,40 In this study, the group of patients with persistent eosinophilia had more severe asthma and logically higher doses of IC. However, these 2 factors were not associated with a greater risk of inflammatory phenotype variability. This finding reveals the existence of a group of patients with eosinophilic inflammatory phenotype in IS who do not respond to treatment with IC and whose asthma is more severe.
The retrospective nature of our study generates limitations, such as: bias in patient selection (this depended on the availability of a second IS test); time between collection of the 2 samples (this differed among patients); some missing clinical data (such as treatment compliance, doses of systemic corticosteroids, time from start of smoking habit and accumulated exposure in pack-years for each individual); and lack of information on secondary variables, such as ACT, FENO, and IgE levels in some cases. However, these limitations did not affect the main results of the study, and sufficient power could be generated to identify 2 factors associated with changes in IS cellularity.
Strengths of the study include a larger sample size than other similar series, and a detailed analysis which included multiple clinical asthma variables, allowing a full clinical characterization of the patients. Sputum samples were induced, processed and analyzed according to a systemized protocol, always by the same, widely experienced technical staff, under rigorous quality control. These aspects helped reduce any potential inter-observer variability in collection and processing of samples and in cell counts.
ConclusionsThe results of our study confirm the existence of a high proportion of asthma patients whose inflammatory phenotype, determined by inflammatory cell counts in IS, changes in serial determinations. The finding that smoking and recent asthma exacerbations contribute to that variability adds new and significant information to our knowledge of this phenomenon. Our data suggest that a recent disease exacerbation should be considered an exclusion criteria for IS testing. In our opinion, these results do not question the utility of IS in the evaluation of asthma patients, but they do limit their application in the clinical setting by identifying some patients in whom the test should be delayed.
Conflict of InterestsThe authors do not have any conflict of interests with the contents of this article.
Please cite this article as: Suárez-Cuartín G, Crespo A, Mateus E, Torrejón M, Giner J, Belda A, et al. Variabilidad del fenotipo inflamatorio del asma en el esputo inducido. Frecuencia y causas. Arch Bronconeumol. 2016;52:76–81.