To describe an outbreak of multidrug-resistant tuberculosis (MDR-TB) in two schools.
MethodsThis was a prospective, observational study of an outbreak of MDR-TB in 2 schools located in the towns of Onda and Nules, in the Spanish province of Castellon, from the moment of detection in November 2008 until November 2014, including patient follow-up and contact tracing.
ResultsFive cases of MDR-TB were diagnosed. Overall attack rate was 0.9%, and among the contacts traced, 66 had latent tuberculous infection, with an infection rate of 14.4%. Molecular characterization of the 5 M. tuberculosis isolates was performed by restriction fragment length polymorphism (RFLP) analysis of the IS6110 sequence. In all 5 patients, cultures were negative at 4-month follow-up, showing the efficacy of the treatment given. No recurrence has been reported to date.
ConclusionsIn the context of globalization and the increased prevalence of MDR-TB, outbreaks such as the one presented here are only to be expected. Contact tracing, strict follow-up of confirmed cases, the availability of fast diagnostic techniques to avoid treatment delay, and chemoprophylaxis, together with the molecular characterization of strains, are still essential.
Descripción de un brote de tuberculosis multirresistente (TB-MDR) en el medio escolar.
MétodosSe ha realizado un estudio prospectivo y observacional de un brote de TB-MDR en 2 colegios de Onda y de Nules de educación secundaria, en la provincia de Castellón, desde su detección en noviembre de 2008 hasta noviembre de 2014, con seguimiento de los casos y estudio de los contactos.
ResultadosSe diagnosticaron 5 casos de TB-MDR, con una tasa global de ataque de la enfermedad del 0,9% y en el estudio de contactos se detectaron 66 con infección latente tuberculosa, con una tasa de infección del 14,4%. Los 5 aislamientos de M. tuberculosis se estudiaron mediante el análisis del polimorfismo de los fragmentos de restricción (RFLP) de la secuencia IS6110 para su caracterización molecular. En los 5 pacientes el cultivo se negativizó a los 4 meses, demostrando la eficacia del tratamiento pautado, sin recaídas hasta la actualidad.
ConclusionesCon la actual globalización y el aumento de la TB-MDR no es extraño la presentación de un brote como el que presentamos y sigue siendo fundamental el estudio de los contactos, el seguimiento estricto de los casos y la disponibilidad de las técnicas de diagnóstico para no demorar el inicio del tratamiento y la quimioprofilaxis, así como la caracterización molecular de las cepas.
In the 1980s, the emergence of HIV/AIDS led to a dramatic increase in the incidence of tuberculosis, primarily in countries with fewer resources, initially sub-Saharan Africa, followed several years later by south-east Asia. In 1993, the World Health Organization (WHO) declared tuberculosis to be a global health emergency.1
Epidemics of multi-drug resistant tuberculosis (MDR-TB), defined as Mycobacterium tuberculosis infection resistant to isoniazid and rifampicin, were first described in the United States and Europe at the beginning of the 90s. MDR-TB occurs mainly in HIV-infected patients, the transmission pattern is explosive, attack rates are high and incubation periods are short due to the immunosuppressed status of the host and the lack of adequate preventive measures against tuberculosis transmission in hospitals and penitentiary institutions affected by these outbreaks. Healthcare personnel are not exempt from risk.2–4
In 1994, the WHO, together with the International Union against Tuberculosis and Lung Disease, set up their Surveillance of Drug Resistance in Tuberculosis program. Until then, drug resistance had been a minor problem, usually related with poor treatment adherence, and primary multi-drug resistance was very rare.
Poverty, overpopulation, lack of economic resources for obtaining second-line drugs, the absence of effective programs for the management of tuberculosis, the HIV epidemic, and moving populations; all are reasons for the appearance and international dissemination of multi-resistant strains of M. tuberculosis. This has become a worldwide health problem, but one that is unequally distributed – countries such as Brazil, China, India, the Russian Federation, and South Africa are much more severely affected.5
Confirmation of diagnosis in a patient with suspected MDR-TB and the considerable delay in starting treatment was an additional problem, since cultures and sensitivity studies had to be performed. This particularly affected the start of second-line treatment.6 The situation has improved, thanks to new techniques that detect M. tuberculosis DNA in respiratory specimens, allowing the diagnosis and detection of isoniazid and/or rifampicin resistance in a matter of hours.7,8
Tuberculosis epidemics in schools are not uncommon, and when they occur, contact tracing is essential to diagnose secondary cases, to initiate prompt treatment of patients and carriers who require it, and to identify the real index case.9
This is a description of an outbreak of MDR-TB in a school setting and the difficulties we encountered in managing both patients with disease and carriers.
MethodsWe conducted an observational, prospective study of an MDR-TB outbreak detected in November 2008 in 2 schools in Onda (O) and Nules (N) in the province of Castellón (Spain). A microepidemic or epidemic outbreak was defined as the appearance of 3 or more cases of tuberculosis related in space and time, or the diagnosis of at least 2 patients generated by the same index case.9,10
The index case was the first patient diagnosed, and the source case, or real index case, was the patient who was most likely to be the origin of the outbreak. A contact was any person who had shared enclosed spaces with a tuberculosis carrier.
After the index case was detected, contact tracing was performed, in accordance with previously published guidelines,9,10 using the concentric circles or “stone-in-the-water” model.11
The Castellón Public Health Center worked with the health centers in O and N to coordinate contact tracing in the 2 schools in which the index case was employed as a teacher, and among family members. They were also responsible for providing information to the parents of pupils at the school.
Tuberculin testing was performed using the Mantoux technique with 2TU PPD RT 23.
Latent tuberculosis infection was defined as a positive Mantoux test with a 5mm induration at 72h. An initial Mantoux test was performed and repeated 2 months later in cases which were initially negative. Individuals aged over 50 years with a negative result repeated the test 7 days later, to determine the booster effect.
All individuals with a positive Mantoux test had a chest X-ray.
If mycobacteria were cultured, the intraspecific differentiation was identified using restriction fragment length polymorphism (RFLP) analysis of the IS6110 sequence.12
Statistical AnalysisTuberculosis infection and disease attack rates were estimated in contacts and compared using Chi-squared and Fisher statistical tests in the different risk groups. Odds ratio (OR) was used to estimate the association between contacts according to exposure to MDR-TB cases and the 95% confidence interval was calculated. P-values <.05 were considered significant and the Epi-info program version 5 was used for all statistical calculations.
ResultsContact TracingThe index case taught second year secondary pupils (10h a week) in school O. The previous year he had taught first year pupils. The 2 secondary cases also taught all year grades in school O; their pupils were aged between 11 and 15 years.
After detecting 3 cases among the teachers, we decided to include all pupils, teachers, and non-teaching staff in the contact tracing. A total of 205 of the 207 secondary school pupils (99%) and 200 of the 205 primary and infant school pupils (97.5%) participated. There was a significant increase in positive Mantoux tests between the 1st year and 4th year of secondary school (P<.001).
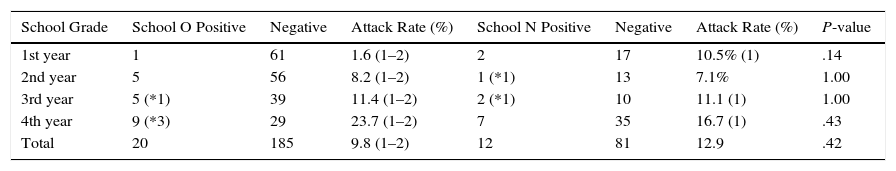
The index case also taught first and third year secondary school pupils in school N (4h a week). All exposed cases participated (93/93, 100%) in contact tracing, including all the pupils that case 1 had taught the previous year. Table 1 shows the results from the pupils of both schools.
Comparison of Mantoux Reaction and Tuberculin Conversion in Schoolchildren in Secondary Schools O and N.
| School Grade | School O Positive | Negative | Attack Rate (%) | School N Positive | Negative | Attack Rate (%) | P-value |
|---|---|---|---|---|---|---|---|
| 1st year | 1 | 61 | 1.6 (1–2) | 2 | 17 | 10.5% (1) | .14 |
| 2nd year | 5 | 56 | 8.2 (1–2) | 1 (*1) | 13 | 7.1% | 1.00 |
| 3rd year | 5 (*1) | 39 | 11.4 (1–2) | 2 (*1) | 10 | 11.1 (1) | 1.00 |
| 4th year | 9 (*3) | 29 | 23.7 (1–2) | 7 | 35 | 16.7 (1) | .43 |
| Total | 20 | 185 | 9.8 (1–2) | 12 | 81 | 12.9 | .42 |
1: Classes taught by the index case in school O (2nd year) and N (1st and 3rd years).
2: Classes taught by the secondary cases; all years in school O.
*: tuberculin conversion.
No significant differences were observed in the risk of developing infection among secondary pupils at both schools [OR 0.63 (95% CI 0.28–1.42), P=.223]; nor was any significant difference observed when pupils attending classes given by the index case were examined separately [OR 0.51 (95% CI 0.15–1.66), P=.22].
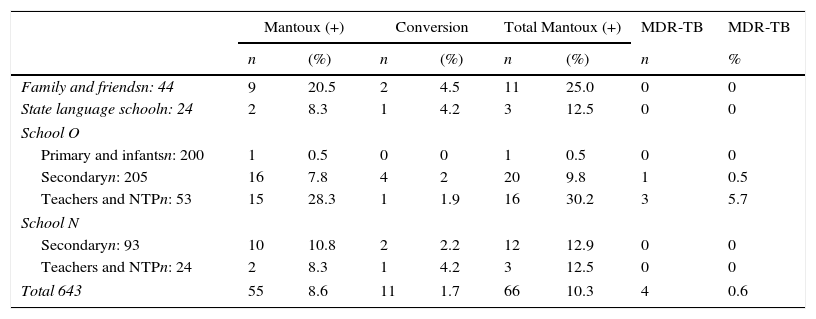
Table 2 shows the results of all contacts that were studied.
Overall Results from Study of Contacts and Cases of MDR-TB.
| Mantoux (+) | Conversion | Total Mantoux (+) | MDR-TB | MDR-TB | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | % | |
| Family and friendsn: 44 | 9 | 20.5 | 2 | 4.5 | 11 | 25.0 | 0 | 0 |
| State language schooln: 24 | 2 | 8.3 | 1 | 4.2 | 3 | 12.5 | 0 | 0 |
| School O | ||||||||
| Primary and infantsn: 200 | 1 | 0.5 | 0 | 0 | 1 | 0.5 | 0 | 0 |
| Secondaryn: 205 | 16 | 7.8 | 4 | 2 | 20 | 9.8 | 1 | 0.5 |
| Teachers and NTPn: 53 | 15 | 28.3 | 1 | 1.9 | 16 | 30.2 | 3 | 5.7 |
| School N | ||||||||
| Secondaryn: 93 | 10 | 10.8 | 2 | 2.2 | 12 | 12.9 | 0 | 0 |
| Teachers and NTPn: 24 | 2 | 8.3 | 1 | 4.2 | 3 | 12.5 | 0 | 0 |
| Total 643 | 55 | 8.6 | 11 | 1.7 | 66 | 10.3 | 4 | 0.6 |
MDR-TB, multiresistant tuberculosis; NTP, non-teaching personnel.
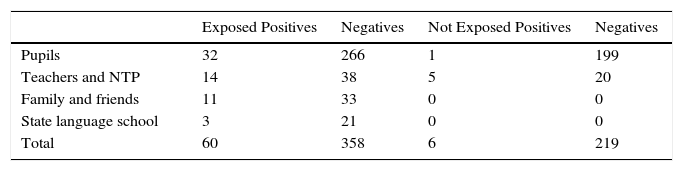
If we compare the risk of infection among teachers from both schools, teachers at school O show a higher risk [OR 3.43 (95% CI 0.76–17.48) P=.069], probably due to greater exposure. It is interesting to note that none of the 3 teachers who contracted the disease had received BCG vaccination, and if vaccinated contacts (10) are compared to unvaccinated contacts (21), the OR of developing the disease is 0.51 (95% CI 0.0–5.13). This difference is not statistically significant (P=.59), but it may indicate some protective effect from vaccination. Table 3 compares the results in cases exposed and not exposed to MDR-TB cases. The OR of contracting infection was 6.12 (95% CI 2.58–17.59), which is statistically significant (P<.001). The overall rate of disease attack was 0.9% and rate of infection 14.4%.
Comparison of Mantoux Reaction and Tuberculin Conversion in Individuals Exposed and Not Exposed to MDR-TB Cases.
| Exposed Positives | Negatives | Not Exposed Positives | Negatives | |
|---|---|---|---|---|
| Pupils | 32 | 266 | 1 | 199 |
| Teachers and NTP | 14 | 38 | 5 | 20 |
| Family and friends | 11 | 33 | 0 | 0 |
| State language school | 3 | 21 | 0 | 0 |
| Total | 60 | 358 | 6 | 219 |
NTP, non-teaching personnel.
Chemoprophylaxis with isoniazid was initially recommended. When isolation of multi-resistant M. tuberculosis was confirmed, adults discontinued isoniazid, but children continued to receive it for another 6 months, after their parents were informed. Clinical and radiological follow-up was recommended for at least 2 years.
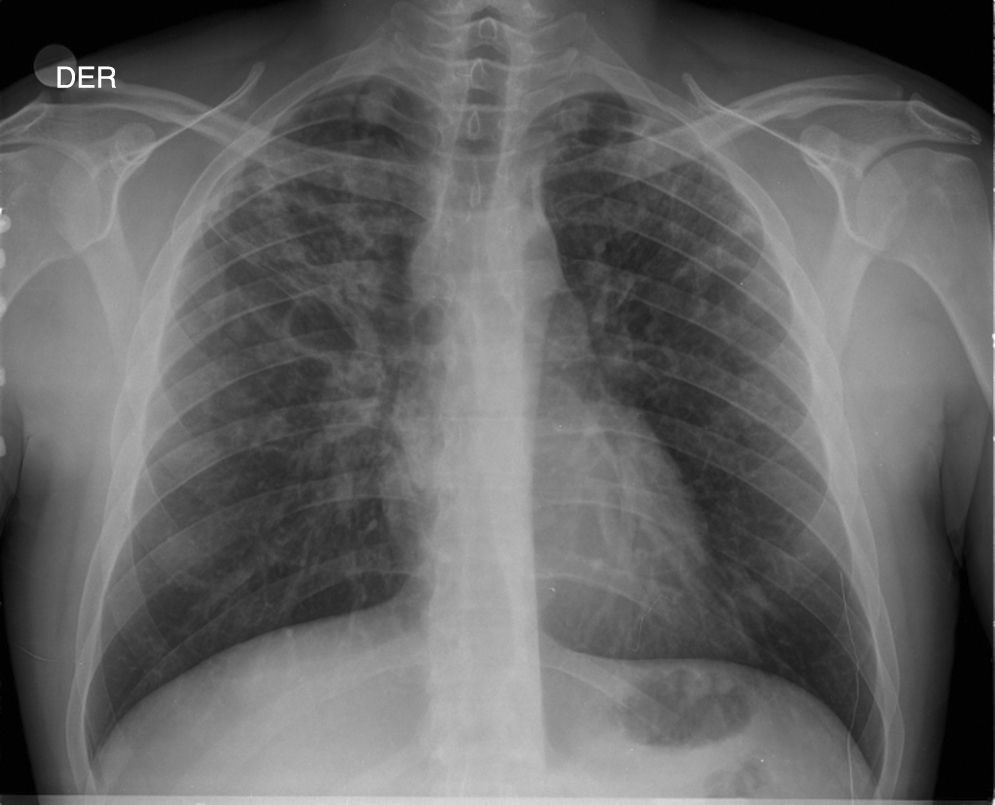
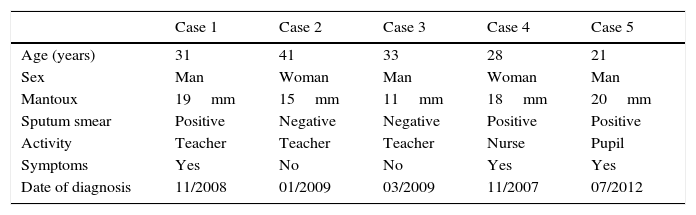
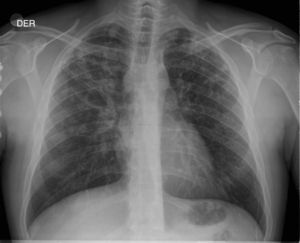
Description of CasesFive cases of MDR-TB were diagnosed. Their clinical characteristics are described in Table 4. It is interesting to observe that the delay in obtaining a microbiological diagnosis in the first case was 4 months, while for the last case, results were received within 72h. This improvement is associated with the introduction of polymerase chain reaction (PCR) techniques in respiratory samples in the reference hospital. In the index case, or case 1, the delay in diagnosis was confounded by an initial interpretation of community-acquired pneumonia (see chest X-ray in Fig. 1).
Clinical Characteristics of Cases of Multiresistant Tuberculosis.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age (years) | 31 | 41 | 33 | 28 | 21 |
| Sex | Man | Woman | Man | Woman | Man |
| Mantoux | 19mm | 15mm | 11mm | 18mm | 20mm |
| Sputum smear | Positive | Negative | Negative | Positive | Positive |
| Activity | Teacher | Teacher | Teacher | Nurse | Pupil |
| Symptoms | Yes | No | No | Yes | Yes |
| Date of diagnosis | 11/2008 | 01/2009 | 03/2009 | 11/2007 | 07/2012 |
All M. tuberculosis isolates obtained from study patients were tested for sensitivity to first-line drugs in the reference laboratory (Hospital General de Castellón). Any strains showing resistance to any drugs were sent to the Carlos III Health Institute in Madrid for further sensitivity studies, and to the Genetics Laboratory of the University of Zaragoza for molecular characterization by RFLP analysis of the IS6100 sequence. Strains with at least 5 copies of the IS6001 sequence were analyzed using spoligotyping. This technique is less discriminative, but it identifies polymorphism in the DR region, containing direct repetitions separated by spacer sequences, which have a wide variability that is used to obtain different hybridization patterns.
Individuals infected with the same strain are included in a cluster. An M. tuberculosis cluster is defined as 2 or more strains whose RFLP patterns reveal an identical number and position of the IS6110 sequence, when there are 5 or more copies, or else 5 copies of identical molecular weight and identical spoligotyping pattern. All our cases had the same spoligotyping pattern, so we could relate them and determine that it was a single strain that had caused the outbreak.
TreatmentWhen case 4 was diagnosed, this was the first time an M. tuberculosis strain had been isolated in our province. The patient did not present any risk factors for MDR-TB, so she started treatment with ethambutol (25mg/kg), moxifloxacin (400mg/day), prothionamide (15mg/kg, up to 1g/day), and capreomycin 1g intramuscularly 5 days a week until acid-fast bacilli sputum smear became negative, for a period of 18 months. When new cases appeared, we suspected and subsequently confirmed that they were caused by the same strain. The satisfactory clinical and microbiological progress of our patient led us to prescribe the same regimen. Side effects included mild gastrointestinal symptoms, and only case 4 developed severe toxic hepatitis, 10 months after starting treatment. Prothionamide was withdrawn and replaced with cycloserine (15mg/kg, up to 1g/day). The patient completed treatment without incidents after 22 months. All patients achieved a negative sputum culture 4 months into treatment.
DiscussionA tuberculosis outbreak in a school setting is a situation of particular concern for public health,13–15,19,20 but outbreaks have also been described in churches,16 bars,17 buses,18 and even in healthcare centers.2–4 In this article, we report an outbreak in 2 schools, which was complicated by the fact that it was caused by a multiresistant strain of M. tuberculosis. Schools are an environment in which outbreaks are likely to occur: contact is continuous, classrooms are poorly ventilated, and diagnosis is delayed.19–21 In countries with high prevalence of MDR-TB, outbreaks in schools are common.21
Case 4 deserves particular attention, since she was diagnosed 1 year before the school outbreak; she presented no risk factors for MDR-TB,22 having never traveled to countries with high rates of multiresistant strains. This was the first case in our province,23 and there was a considerable delay (4 months) in diagnosis before definitive results were received from the reference laboratory. The patient had had contact with case 1, but case 1 could not attend the contact tracing visit. This underlines the importance of well-designed anti-tuberculosis programs,9,10 since an earlier diagnosis of case 1 may have prevented the outbreak. The index case had done voluntary work in Colombia for 2 summers, and we believe that he may have been infected there. Case 1 infected case 4, and subsequently triggered the school outbreak, so case 1 is considered as the source or real index case. Diagnosis of case 1 was delayed by 2 months, which meant that the schoolchildren and teaching staff started chemoprophylaxis treatment with isoniazid. When drug sensitivity study results were received, isoniazid had to be discontinued. A meeting was held with the parents, and we explained that, in multiresistant cases, no effective drug was available to prevent the infection from developing to tuberculous disease.10 This generated great anxiety and concern, so after several meetings coordinated by the Public Health Center, we decided to continue clinical and radiological monitoring for at least 2 years in Mantoux-positive cases, as indicated in the guidelines.10 We offered optional isoniazid, being the less toxic choice in children, in view of the possibility that individuals may be infected with sensitive strains. Perhaps the follow-up period should have been extended, since case 5 was diagnosed after 3 years. Very few studies have been published that confirm the efficacy of treating latent MDR-TB infection, but adverse events do seem to be an issue.24 Attamma et al. followed up 476 contacts of patients with MDR-TB over a mean duration of 6 years. Patients received isoniazid or a combination of ciprofloxacin and pyrazinamide, and 387 received no treatment. They did not detect any case of MDR-TB, thus supporting the clinical monitoring approach.25 The situation has changed since then, since effective chemoprophylaxis with fluoroquinolones has been reported in studies following MDR-TB cases for periods of up to 12 months.26 However, reluctance to use fluoroquinolones in children and teenagers persists, in view of their adverse effects on growing cartilage.
The rate of infection among exposed cases was 14.4%. This is relatively low, compared to other reports of 30%–100% in school outbreaks caused by sensitive M. tuberculosis.14,15 The same is true of the 0.9% rate of disease, which is much lower than other reported MDR-TB outbreaks that were highly explosive, probably due to co-infection with HIV.2–4 Kritski et al.27 also reported a rate of 8% in a contact tracing study in MDR-TB, and higher rates have also been reported in school outbreaks of M. tuberculosis.14,15 This finding concurs with the observation that multiresistant strains cause less secondary cases than sensitive strains,28 but for epidemiological purposes, we cannot assume that multiresistant strains are less infectious or less virulent than sensitive strains.29
In our study, the BCG vaccine conferred some protective effect among teachers, as previously noted by Kritski et al.27
After diagnosis, treatment was started and cases were closely monitored in monthly clinical check-ups with blood tests and sputum studies, since patients on second-line drugs often encounter toxicity and need encouragement to comply.30,31 All patients achieved a negative sputum culture within 4 months of starting treatment, and no relapses have been seen to date. This is probably because our patients were immunocompetent, non-HIV carriers, and had a good prognosis for treatment.21,32
A very important aspect of the study of outbreaks is genotyping of the causative strains. This information may help determine transmission mechanisms and determine if the disease is due to endogenous reactivation or exogenous reinfection, if it is found to be caused by more than 1 strain of M. tuberculosis. Genotyping of a strain is also a starting point for retrospective contact tracing.2,14,33–35
One limitation of our study is that we could not characterize latent tuberculous infection with interferon gamma release assays (IGRAS), which would have provided greater specificity to Mantoux reactions and tuberculin conversions.
To conclude, we report the first outbreak of MDR-TB in a school setting in Spain. A meticulous study of cases and contacts is crucial, and these individuals must be followed up closely and clearly informed, as the situation can generate considerable anxiety. As globalization continues, we can expect to encounter more tuberculosis caused by multiresistant strains. New technologies must be put to work to provide rapid diagnoses and to guide the right therapeutic intervention.
Contribution of the AuthorsDr. Miravet contributed to the follow-up of cases, data acquisition, analysis and interpretation, manuscript preparation and the review of the final version submitted for evaluation; Dr. García participated in the follow-up of cases and the review of the final version submitted for evaluation; Dr. Arnedo participated in contact tracing, data acquisition and interpretation, manuscript preparation and the review of the final version submitted for evaluation; Dr. Bellido participated in contact tracing, data analysis and interpretation, and the review of the final manuscript submitted for evaluation; Dr. Romeu contributed to contact tracing and the review of the final manuscript submitted for evaluation; Dr. Gil contributed to the microbiological study and the review of the final manuscript submitted for evaluation; Dr. Cortés participated in the follow-up of cases, and contributed to the review of the final manuscript submitted for evaluation.
Conflict of InterestsThe authors declare that they have no conflict of interests.
We thank Dr. Caminero Luna for his suggestions regarding patient treatment.
Please cite this article as: Sorribes LM, Pena AA, Blasco JBB, García MAR, Fortuño MG, Sidro PG, et al. Brote de tuberculosis multirresistente en dos colegios de educación secundaria. Arch Bronconeumol. 2016;52:70–75.