To assess the linguistic equivalence of the COPD Assessment Test versions in Catalan (CAT-C), Galician (CAT-G) and Basque (CAT-V) with the validated Spanish version (CAT) in COPD patients able to use both official languages.
MethodsStudy performed in primary care centers in Catalonia, Galicia and the Basque Country. Half of the patients completed the questionnaire in their local language followed by the CAT in Spanish, while the other half did so in reverse order.
Results151 COPD patients were included in the study, with a mean age of 69.0 (SD: 9.7) years. Most (79.5%) were men, 11.3% were educated up touniversity level, and 31.8% were current smokers. Mean FEV1 was 61.4% (SD: 16.8) predicted and 83.9% of patients were GOLD grade I or II. Concordance between CAT-C, CAT-G and CAT-V and CAT was high, with differences between scores from 0.4 to −0.5. Reliability (Cronbach's alpha) ranged from 0.72 to 0.86. Convergent validity, when correlated with the Medical Research Council Dyspnea scale (P=.003) and Saint George's Respiratory Questionnaire (ICC, r=0.74) scores, was significant.
ConclusionsCAT-C, CAT-G and CAT-V scores were similar to CAT scores, with high correlation and concordance. These results show the equivalence between the validated Spanish CAT and the version in other languages, so CAT-C, CAT-G and CAT-V can be used in individuals whose main language is Catalan, Galician or Basque.
Evaluar la equivalencia lingüística de las versiones del cuestionario COPD Assessment Test en catalán (CAT-C), gallego (CAT-G) y vasco (CAT-V) con la versión en castellano (CAT) en pacientes con enfermedad pulmonar obstructiva crónica que hablan indistintamente las 2 lenguas cooficiales.
MétodosEstudio realizado en centros de atención primaria de Cataluña, Galicia y País Vasco. La mitad de los pacientes completó primero el CAT en castellano seguido del CAT en la lengua correspondiente de este estudio; la otra mitad lo hizo en orden inverso.
ResultadosSe incluyó a 151 pacientes con edad media (DE) de 69,0 (9,7) años. Un 79,5% eran hombres, el 11,3% tenía estudios universitarios y el 31,8% eran fumadores. El valor medio (DE) de la FEV1 fue del 61,4% (16,8) del valor de referencia. La mayoría de pacientes (83,9%) tenía un grado GOLD I/II de limitación al flujo aéreo. La concordancia entre las puntuaciones de las diferentes versiones del CAT fue alta, con diferencias entre 0,4 y –0,5 puntos. Su fiabilidad (Cronbach) fue de 0,72-0,86. La validez convergente al correlacionar con el nivel de disnea de la escala Medical Research Council Dyspnea scale (p=0,003) y con el Saint George's Respiratory Questionnaire (ICC, r=0,74) fue significativa.
ConclusionesLas puntuaciones obtenidas con CAT-C, CAT-G y CAT-V fueron similares a las de CAT, con una alta correlación y concordancia entre ellas. Estos resultados muestran la equivalencia entre las versiones de CAT en diferentes lenguas e indican que este cuestionario puede ser utilizado indistintamente en pacientes cuya lengua principal sea catalán, gallego o vasco.
Chronic obstructive pulmonary disease (COPD) is one of the main causes of morbidity and mortality worldwide.1 In Spain, it affects approximately 10% of the general population aged between 40 and 80 years.2
The Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) Strategy for Diagnosis, Management and Prevention of COPD recommends that drug treatment be guided by a combined evaluation of the patient's severity of airflow limitation (FEV1), prior history of exacerbations, and symptoms.1 To this end, GOLD recommends the use of validated questionnaires, such as the Medical Research Council Dyspnea scale (MRC),3 the COPD Assessment Test (CAT)4 or the Clinical COPD Questionnaire (CCQ).5 The CAT questionnaire, specifically, has been adapted and validated in over 60 languages and dialects throughout the world, including Spanish.6
In general terms, a person is considered bilingual if they are competent in 2 languages and can easily switch from one to the other.7,8 Spain is a country in which inhabitants in some autonomous communities are bilingual in 2 official languages, primarily Catalonia, Galicia, and the Basque Country, which have the highest percentage of speakers of languages other than Spanish.9,10 In Catalonia, for example, according to the 2013 linguistic census of the Statistical Institute of Catalonia (IDESCAT), Catalan was the habitual language of 36.3% of the overall population, and this proportion rose to more than 60% in most regions of the province11; in Galicia, according to data from the Statistical Institute of Galicia, 33.9% of the population said that they were most comfortable speaking Galician, and 29% read Galician and Spanish equally competently12; in the Basque Country, the Basque Country Sociolinguistic Survey (ESE), conducted by the Department of Education, Linguistic Policy and Culture of the Basque Government, found that 22.6% of the population consider themselves Basque speakers or use Basque and Spanish indiscriminately.13 These data reflect the situation in each autonomous community, but it must be remembered that Catalan and Basque, in particular, are also spoken in other regions, such as the Balearic Islands, in the case of Catalan, or in Navarre, in the case of Basque, so the use of these languages is even more common and more geographically widespread.
The aim of this study was to evaluate the linguistic equivalence between the CAT questionnaire in Spanish and its versions in 3 of the other official languages spoken in Spain: Catalan, Galician and Basque. These versions were prepared using a specific methodology (translation-backtranslation).14 Our second objective was to evaluate the measurements and psychometric properties of each of the versions.
MethodTranslation-BacktranslationBefore starting the study, the CAT questionnaire was translated and adapted culturally to Catalan, Galician and Basque using a translation-backtranslation process14 that involved 2 direct translations by professional bilingual translators, which were reviewed and consolidated in a single, consensus version. This consensus version was backtranslated to Spanish by another professional bilingual translator, and this backtranslation was compared with the original to detect any differences. Finally, the single version in each target language was completed by 10–15 COPD patients in each language. The items from the Spanish version and the translated and adapted versions are included in Table 1.
CAT Questionnaire Items of the Versions in Spanish, Catalan, Galician and Basque.
| CAT | CAT-C | CAT-G | CAT-B | |
|---|---|---|---|---|
| Item 1 | Nunca toso- Siempre estoy tosiendo | Mai no tusso- Sempre estic tossint | Non tuso nunca- Sempre estou tusindo | Ez dut inoiz eztulik egiten-Beti eztulka ari naiz |
| Item 2 | No tengo flema (mucosidad) en el pecho- Tengo el pecho completamente lleno de flema (mucosidad) | No tinc flegma (mucositat) al pit-Tinc el pit completament ple de flegma (mucositat) | Non teño flegma (mucosidade) no peito- Teño o peito completamente cheo de flegma (mucosidade) | Ez daukat flemarik (mukirik) bularrean- Bularra erabat flemaz (mukiz) beteta daukat |
| Item 3 | No siento ninguna opresión en el pecho-Siento mucha opresión en el pecho | No sento cap opressió al pit- Sento molta opressió al pit | Non sinto opresión ningunha no peito- Sinto moita opresión no peito | Bular aldean ez dut inolako estutasunik- Bular aldean estutasun handia dut |
| Item 4 | Cuando subo una pendiente o un tramo de escaleras, no me falta el aire- Cuando subo una pendiente o un tramo de escaleras, me falta mucho el aire | Quan pujo un pendent o un tram d’escales, no em falta l’aire- Quan pujo un pendent o un tram d’escales, em falta molt l’aire | Cando subo unha pendente ou un tramo de escaleiras non me falta o aire- Cando subo unha pendente ou un tramo de escaleiras fáltame moito o aire | Maldan gora edo eskaileretan gora noanean, ez zait arnasa estutzen- Maldan gora edo eskaileretan gora noanean, arnas-estuka jartzen naiz |
| Item 5 | No me siento limitado para realizar actividades domésticas- Me siento muy limitado para realizar actividades domésticas | No em sento limitat/da per realitzar activitats domèstiques-Em sento molt limitat/da per realitzar activitats domèstiques | Non me sinto limitado/a para realizar actividades domésticas- Síntome moi limitado/a para realizar actividades domésticas | Ez daukat arazorik etxeko lanak egiteko- Ezinean ibiltzen naiz etxeko lanak egiteko |
| Item 6 | Me siento seguro al salir de casa a pesar de la afección pulmonar que padezco- No me siento nada seguro al salir de casa debido a la afección pulmonar que padezco | Em sento segur/a en sortir de casa malgrat l’afecció pulmonar que pateixo- No em sento gens segur/a en sortir de casa a causa de l’afecció pulmonar que pateixo | Síntome seguro/a ao saír da casa a pesar da afección pulmonar que padezo- Non me sinto nada seguro/a ao saír da casa pola afección pulmonar que padezo | Seguru sentitzen naiz etxetik irtetean, biriketako gaixotasuna izan arren- Ez naiz inondik ere seguru sentitzen etxetik irtetean, biriketako gaixotasuna dela-eta |
| Item 7 | Duermo sin problemas- Tengo problemas para dormir debido a la afección pulmonar que padezco | Dormo sense problemes- Tinc problemes per dormir a causa de l’afecció pulmonar que pateixo | Durmo sen problemas- Teño problemas para durmir debido á afección pulmonar que padezo | Ez dut arazorik lo egiteko- Lo egiteko arazoak ditut, biriketako gaitza dela-eta |
| Item 8 | Tengo mucha energía- No tengo ninguna energía | Tinc molta energia- No tinc gens d’energia | Teño moita enerxía- Non teño enerxía ningunha | Indartsu sentitzen naiz- Ez dut batere indarrik |
This was an observational, multicenter study in which patients were enrolled consecutively. Study data were collected in a single visit. All questionnaires were self-administered. The order of completion of the CAT questionnaire alternated: patients with odd code numbers completed the Spanish version first, followed by the Catalan (CAT-C), Galician (CAT-G) or Basque (CAT-B) version, depending on the patient's autonomous community, and patients with even code numbers completed the questionnaires in the inverse order. Participants also completed the Saint George's Respiratory Questionnaire (SGRQ) on quality of life and the London Chest Activity of Daily Living scale (LCADL), both adapted for the Spanish population.15,16 Sociodemographic and clinical data, including comorbidities (cardiovascular, metabolic, neurological and musculoskeletal diseases, and mood/psychiatric disorders), dyspnea grade (MRC scale), presence and number of exacerbations, and COPD grade and level of severity according to GOLD criteria1 were also recorded.
PatientsThe study was performed in patients aged 40 years or older, diagnosed with COPD at least 6 months before study inclusion (post-bronchodilator FEV1/FVC<0.7), with a current or past smoking history of more than 10 pack-years, and clinically stable COPD in at least the previous 3 months. Subjects were excluded if they presented other chronic respiratory diseases that might confound study results or if they had cognitive disorders that could affect their ability to complete the questionnaires. Study patients were also required to be completely fluent, both written and spoken, in the 2 official languages (Spanish and Catalan, Galician or Basque). The study was performed simultaneously in 2 primary care centers in Catalonia, Galicia and the Basque Country, with the participation of physicians who regularly treated COPD patients.
Ethical AspectsThe study protocol was reviewed and approved by accredited ethics committees, and participants gave written informed consent. The study was performed in compliance with the ethical and confidentiality requirements applicable to this type of study.
Data AnalysisTo evaluate the concordance of each of the linguistic versions with the CAT, a total of 50 patients were required for each language. This sample size would be sufficient to detect intraclass correlation coefficients (ICC) greater or equal to 0.5 (moderate concordance), assuming a minimum ICC of 0.2, with a level of significance of 0.05 and a statistical power of 0.80.
Concordance between the CAT-C, CAT-G and CAT-B with the CAT was analyzed using the ICC for the overall questionnaire score, and the weighted kappa statistic was used for individual items. Kappa statistic reference values were: <0.20, poor; 0.21–0.40, weak; 0.41–0.60, moderate; 0.61–0.80, good, and 0.81–1.00, very good.17
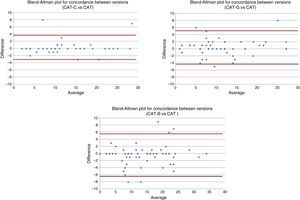
Possible deviations in extreme values are presented in the Bland–Altman plots in Fig. 1.
Reliability was analyzed in terms of internal consistency, using Cronbach's alpha. Construct validity was evaluated in terms of discriminant and convergent validity. To determine discriminant validity, the correlation between the scores of each of the CAT versions and clinical variables was analyzed using the Pearson correlation coefficient and analysis of variance (ANOVA). For convergent validity, the correlation between the scores of the CAT questionnaires and the SGRQ questionnaire, the LCADL scale and the question on patient-perceived state of health was evaluating using the Pearson correlation coefficient and the ANOVA. Finally, questionnaire feasibility was evaluated by calculating the rate of non-completion, time to administer, and the percentage of patients with ceiling effect (maximum score on the questionnaire) and floor effect (minimum score on the questionnaire).
ResultsPatient Characteristics (Table 2)Table 2 includes the 48, 48 and 55 evaluable patients in Catalonia, Galicia and the Basque Country, respectively. Their mean age (standard deviation, SD) was 69.0 (9.7) years, 79.5% were men and 11.3% had studied to university level; 31.8% of patients smoked 37.3 (13.6) pack-years and 68.2% were former smokers of 38.3 (23.7) pack-years. Post-bronchodilator spirometry showed FEV1 values of 64.2% (17.6), and FEV1/FVC of 60.3% (9.0). In total, 24.8% of patients had mild COPD (GOLD grade I), 59.1% moderate COPD (GOLD grade II) and 16.1% had severe/very severe COPD (GOLD grades III–IV). With regard to GOLD groups, 29.1% were in group A, 50.3% in group B, 2.6% in group C, and 17.9% in group D. On the MRC scale, 18% had grade 1 dyspnea, 56.7% had grade 2, 21.3% grade 3, and 4% grade 4.
Sociodemographic Characteristics and Lung Function Parameters.
| Catalonia | Galicia | Basque Country | Total | ||
|---|---|---|---|---|---|
| Age (years) | Mean (SD) | 71.6 (9.3) | 65.9 (9.3) | 69.5 (9.7) | 69.0 (9.7) |
| Sex | Male | 89.6% | 89.6% | 61.8% | 79.5% |
| Female | 10.4% | 10.4% | 38.2% | 20.5% | |
| Educational level | Lower than primary | 6.3% | 27.1% | 14.8% | 16.0% |
| Primary | 41.7% | 56.3% | 38.9% | 45.3% | |
| Secondary | 29.2% | 12.5% | 29.6% | 24.0% | |
| University | 12.5% | 4.2% | 16.7% | 11.3% | |
| Not known | 10.4% | 0.0% | 0.0% | 3.3% | |
| Comorbidities | 75.0% | 91.7% | 78.2% | 81.5% | |
| FVC (%), mean (SD) | 66.7 (12.1) | 78.4 (20.6) | 77.3 (17.3) | 74.2 (17.7) | |
| FEV1 (%), mean (SD) | 60.1 (16.4) | 63.7 (17.5) | 60.4 (19.5) | 61.4 (16.8) | |
| FEV1/FVC (%), mean (SD) | 62.9 (6.6) | 59.3 (7.7) | 57.4 (10.0) | 59.8 (8.5) | |
| GOLD criteria | GOLD I (mild) | 31.2% | 25.6% | 18.5% | 24.8% |
| GOLD II (moderate) | 56.3% | 63.8% | 57.4% | 59.1% | |
| GOLD III–IV (severe-very severe) | 12.5% | 10.6% | 24.1% | 16.1% | |
| Severity groups | Group A | 29.2% | 35.4% | 23.6% | 29.1% |
| Group B | 58.3% | 41.7% | 50.9% | 50.3% | |
| Group C | 2.1% | 6.3% | 0.0% | 2.6% | |
| Group D | 10.4% | 16.7% | 25.5% | 17.9% | |
| MRC dyspnea scale | Grade 1 | 19.1% | 20.8% | 14.5% | 18.0% |
| Grade 2 | 51.1% | 56.3% | 61.8% | 56.7% | |
| Grade 3 | 25.5% | 20.8% | 18.2% | 21.3% | |
| Grade 4 | 4.3% | 2.1% | 5.5% | 4.0% | |
| Grade 5 | 0.0% | 0.0% | 0.0% | 0.0% |
In total, 21.5% reported exacerbations in the previous 6 months, requiring a change in medication in 37.9% (89.7% received antibiotics and/or corticosteroids, and 31.0% required hospitalization). Mean (SD) number of exacerbations in the previous 6 months was 0.3 (0.6) exacerbations. Comorbidities were observed in 81.5% of patients, in particular cardiovascular diseases (57.0%) and metabolic diseases (39.1%); no statistically significant relationship was found between comorbidities and scores obtained on the different versions of the CAT questionnaire.
CAT Questionnaire Validation in Catalan, Galician and BasqueEquivalence (Table 3)The mean difference (SD) between the CAT questionnaire score and the versions in Catalan, Galician and Basque was 0.3 (1.8); 0.4 (2.4), and –0.5 (3.0) points, respectively (Table 3). None of these differences was statistically significant. ICCs were 0.963, 0.942 and 0.929, respectively. The Bland–Altman plot did not reveal any systematic bias between the version in Spanish and the different linguistic versions (Fig. 1).
Mean Scores (SD) Obtained in the CAT Questionnaire in the Spanish Version and the Translated Versions (Equivalence).
| CAT Spanish | CAT Translation Version | Difference | 95% CI | P | |
|---|---|---|---|---|---|
| Catalonia | 12.2 (6.6) | 12.5 (6.8) | 0.3 | (−0.2; 0.8) | .509 |
| Galicia | 11.3 (6.9) | 11.7 (7.2) | 0.4 | (0.3; 1.1) | .252 |
| Basque Country | 14.2 (7.9) | 13.7 (8.2) | −0.5 | (1.3; 0.4) | .193 |
Concordance between the CAT and CAT-C questionnaires was very good for all items (kappa>0.800 in all questions). The version in Galician showed a moderate concordance in question 6 (kappa=0.577), while good levels of concordance were achieved for the individual items according to the weighted kappa statistic (kappa≥0.644), with the exception of question 8, that only reached a 50% level of concordance with the CAT questionnaire. The level of concordance between the CAT and CAT-B questionnaires was good or very good for all items (kappa between 0.678 and 0.852).
ReliabilityThe CAT questionnaire and the other versions showed good internal consistency (Cronbach's alpha). The CAT-C obtained a coefficient of 0.796, the CAT-B obtained the highest value (coefficient=0.861), and the CAT-G the lowest (coefficient=0.721).
Discriminant ValidityThe ability of the CAT questionnaires in the Catalan, Galician and Basque linguistic versions to correlate with different clinical aspects of patients varied, depending on the version and the clinical parameter, although it was generally acceptable. The CAT-C questionnaire was the only one that correlated with the presence of exacerbations, and only exacerbations in the 6 previous months, with mean scores (SD) in patients with and without exacerbations of 11.5 (5.9) and 22.0 (8.5), respectively (P=.019) (Table 4), while CAT-C and CAT-B discriminated well between patients in terms of severity of airflow limitation (GOLD grades). All linguistic versions also correlated with the GOLD severity groups; the greatest correlation was found for group C/D, except for the CAT-G, which correlated best with group B (P<.001 in all comparisons). The CAT-G and CAT-B questionnaires discriminated well between grades of dyspnea (MRC scale) (P=.010 and P=.012, respectively), while in the CAT-C, the score was higher for dyspnea grade 1 than for grade 2, so discrimination was rather poor (Table 5). Finally, post-bronchodilator FEV1 (%) and FEV1/FVC (%) showed a statistically significant correlation with the scores in the different versions of the CAT questionnaire, while no correlation was found for FVC (%) (Table 6).
Relationship Between Mean Scores (SD) in the CAT Questionnaire Versions and Presence of Exacerbations in the 6 Months Before the Study Visit (Discriminant Validity).
| Exacerbations in Previous 6 Monthsa | Correlation Between Scores and Exacerbations | ||||
|---|---|---|---|---|---|
| No | Yes | P | r | P | |
| CAT-C | 11.5 (5.9) | 22.0 (8.5) | .019 | 0.278 | .062 |
| CAT-G | 11.6 (6.9) | 12.0 (7.8) | .836 | −0.035 | .813 |
| CAT-B | 12.8 (7.7) | 17.5 (9.4) | .089 | 0.242 | .075 |
| CAT | 12.0 (6.6) | 14.4 (9.0) | .186 | 0.104 | .205 |
r=Spearman's correlation coefficient.
Correlation Between Scores of CAT Questionnaire Versions, GOLD Criteria, COPD Severity Groups, and Dyspnea Grade (Discriminant Validity).
| GOLD Criteria | P | Severity Group | P | Dyspnea Grade (MRC Scale) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III–IV | A | B | C/D | 1 | 2 | 3–4 | ||||
| CAT-C | 9.7 (4.8) | 13.0 (7.0) | 17.2 (8.1) | .059 | 6.5 (3.4) | 14.5 (6.0) | 17.2 (8.1) | .001 | 10.8 (2.6) | 9.9 (5.6) | 16.6 (6.8) | .003 |
| CAT-G | 9.3 (3.8) | 12.6 (7.7) | 13.4 (10.1) | .351 | 6.9 (3.0) | 15.8 (6.1) | 11.8 (9.2) | .001 | 7.2 (4.4) | 11.5 (6.2) | 16.5 (9.0) | .010 |
| CAT-B | 9.7 (5.5) | 12.6 (8.2) | 18.4 (7.6) | .022 | 5.1 (2.7) | 14.9 (6.8) | 19.3 (8.1) | .001 | 9.0 (4.8) | 12.8 (7.9) | 19.0 (8.5) | .012 |
| CAT | 9.4 (5.0) | 12.9 (7.3) | 16.5 (7.9) | .001 | 5.6 (2.6) | 15.3 (5.6) | 16.1 (8.6) | .001 | 9.3 (4.2) | 11.7 (6.9) | 16.6 (7.8) | <.001 |
Correlation Between Scores Obtained in the CAT Questionnaire Versions with Lung Function (Divergent Validity) and With the SGRQ and LCADL Questionnaires (Convergent Validity).
| CAT-C | CAT-G | CAT-B | CAT | |
|---|---|---|---|---|
| FVC (%) | ||||
| R | −0.260 | −0.219 | −0.160 | −0.163 |
| P | .074 | .134 | .252 | .046 |
| FEV1(%) | ||||
| R | −0.257 | −0.363 | −0.236 | −0.223 |
| P | .078 | .011 | .093 | .006 |
| FEV1/FVC (%) | ||||
| R | −0.239 | −0.315 | −0.196 | −0.190 |
| P | .101 | .029 | .159 | .020 |
| SGRQ | ||||
| R | 0.603 | 0.855 | 0.709 | 0.736 |
| P | <.001 | <.001 | <.001 | <.001 |
| LCADL | ||||
| R | 0.175 | 0.420 | 0.251 | 0.357a |
| P | .256 | .003 | .067 | <.001 |
R=Spearman's correlation coefficient.
Convergent validity was also positive in general, and scores of all CAT questionnaire versions correlated significantly (P<.001) with all dimensions of the SGRQ and with its total score, thus confirming the validity of all of the versions of the CAT questionnaire.
The physical activities dimension score of the LCADL obtained statistically significant coefficients with all versions of the CAT questionnaire, the highest being 0.534 achieved by the CAT-G questionnaire (P<.001) (Table 6).
FeasibilityAll patients fully completed the questionnaires, except for 1 who did not complete the CAT-G questionnaire. A single case of floor effect was observed in the CAT-B and CAT questionnaires, but no ceiling effect was found. Time to administer was short in all cases, with mean values ranging between 128 and 175 seconds. Comparison of the time needed to complete the questionnaire revealed a small but statistically significant difference only between the CAT and the CAT-B, with a mean difference (SD) of 15.6 (48.8) seconds (P=.043).
DiscussionThe results of this study demonstrate the linguistic equivalence and validity of the versions in Catalan, Galician and Basque with the previously validated version in Spanish.
The term “bilingualism” refers to the ability to use 2 languages indiscriminately.8 Bilingual individuals can adopt and understand concepts, values, attitudes and expectations in the 2 languages that they master, so bilingual patients represent a population whose responses should not be automatically extrapolated to the monolingual population.18 For this reason, it is important that a patient's experience with respect to their disease and the description of their resulting state of health can be expressed in the language in which they feel most comfortable, even if they are bilingual.
The results of this study show that there is a good correlation between the scores in each of the translated versions of the CAT (Catalan, Galician and Basque) and the Spanish version6 (previously validated and equivalent to the English version). When the validity of each of the new versions of the CAT questionnaire was evaluated, similar results to those obtained from the Spanish version were obtained. The high internal consistency observed with all the CAT versions (between 0.72 and 0.86) is clearly higher than the accepted threshold for these types of instrument,19 confirming the reliability of the versions. Internal consistency is also in line with the values obtained for the original CAT in English4 and the Spanish version.6 Like the Spanish version of the CAT, the 3 versions studied showed good correlation with lung function measurements and LDADL scores, and a statistically significant association with the severity groups defined in the study and the perception of dyspnea evaluated using the MRC scale, primarily in the Galician and Basque versions; this relationship between the CAT and dyspnea was previously confirmed in studies such as that of Okutan et al.,20 although those authors only found statistically significant differences for dyspnea grades 1 and 2, while in this study, the CAT-G and the CAT-B discriminated well among all MRC grades. Correlation with the SGRQ was not only good, but also the correlation coefficients with the total score on this questionnaire were very similar to those described in previous studies.21,22
We should point out that the 3 versions of the CAT did not detect the same clinical differences among patients, and that only the CAT-C discriminated between the presence of exacerbations, and only exacerbations in the 6 previous months. This aspect must be further investigated by administering the questionnaire to a larger study sample, since in previous studies the CAT was found to be sensitive to differences in COPD stability.6,20 Nor did the versions show the same discriminatory power for airflow limitation, as the CAT-G was the only one that did not show differences according to GOLD grades. Despite the fact that all the versions of the CAT questionnaire distinguished between dyspnea grades (MRC scale) to a statistically significant degree, the CAT-C only showed differences between grades 1–2 and grades 3–4. These differences may be because the questionnaires were administered to 3 heterogeneous populations. Although we attempted to obtain a uniform sample by using the same selection criteria in the 3 populations, some differences emerged: for example, a considerably greater number of men than women were included, particularly in Catalonia and Galicia. This is probably because no methods were employed to ensure the homogeneity of the sample, either at the time of patient selection (e.g., gender quotas), nor were statistical techniques subsequently used to minimize those biases, since a comparison of the 3 populations was not included in the study objectives.
When feasibility was examined, the 3 versions showed satisfactory results with slight differences in time to administer between the version in Basque and the version in Spanish, but this difference of only 15 seconds was not substantial.
Other limitations of this study include the fact that, although the number of patients in each group is sufficient to meet the primary objective, i.e., evaluation of the equivalence of each version with the Spanish version of the CAT, the sample size may be limited for drawing conclusions regarding the validity of the versions studied and subgroup analysis, so the validation data must be taken as exploratory, and the values that show statistical significance must be interpreted with caution. Moreover, although feasibility of all of the versions of the CAT was good, patients completed the questionnaires knowing that they were participating in a research project, so the response rates in standard clinical practice conditions may be lower.
In conclusion, these results show that the versions of the CAT questionnaire in Catalan, Galician and Basque are equivalent to the version in Spanish, so they can be considered as valid for use in the evaluation of the impact of COPD symptoms on patients’ lives. These are tools that do not only help to improve the doctor–patient interaction by allowing a patient to express their health situation in the language in which they feel most comfortable, but they also help patients evaluate their symptoms by letting them chose to complete the questionnaire in the language that is easiest for them to read and understand.
FundingThis study was funded by GlaxoSmithKline and IMS Health received funding from GlaxoSmithKline for their contribution (etrack no. 113047).
Authorship1. Study conception and design: Álvar Agustí, Alberto Fernández-Villar, Alberto Capelastegui, Manuel García-Losa, Guadalupe Sánchez.
2. Data collection: Concepción Nogueiras, Cristóbal Esteban, Jaime González, Juan Luis Mendía, Lourdes Arregui, María Inmaculada Gorordo, Teresa Genover, Francisco Tomas Sesma.
3. Data analysis and interpretation: Álvar Agustí, Alberto Fernández-Villar, Alberto Capelastegui, Beatriz Velasco and Guadalupe Sánchez.
4. Writing, review and approval of the submitted manuscript: Álvar Agustí, Alberto Fernández-Villar, Alberto Capelastegui, Manuel García-Losa, Beatriz Velasco, Guadalupe Sánchez.
Conflict of InterestsAC and JF have no conflict of interests. AA has participated in advisory boards for GSK, Novartis, Chiesi, Almirall, Takeda and Roche. AA has received speaker's honoraria, including participation in boards for GSK, Novartis, Chiesi, Almirall, Takeda and Astra-Zeneca. BV and GS are employees of GSK and have shares in GSK. MGL was an employee of GSK and has shares in GSK.
The authors thank all investigators who participated in the study. The names and affiliations of the investigators are listed in Annex 1. The authors would also like to thank IMS Health for monitoring the study and collaborating with the authors in producing the report and manuscript drafts.
List of participating investigators and experts
Pulmonologists
Alvar Agustí, Institut del Tórax, Hospital Clínic. IDIBAPS, Universitat de Barcelona, Centro de Investigación Biomédica, Servicio de Neumología.
Alberto Capelastegui, Hospital de Galdakao-Usansolo, Galdakao (Bizkaia).
Alberto Fernández-Villar, Servicio de Neumología, Estructura Organizativa de Gestión Integrada de Vigo, IBIV, Vigo (Pontevedra).
Cristobal Esteban, Servicio de Neumología, H. Galdakao.
María Inmaculada Gorordo Unzueta, Servicio de Neumología, H. Galdakao.
Primary care physicians
Concepción Nogueiras, C.S. Val Minor.
Jaime Gonzalez Rey, C.S. Matamá de Vigo,
Juan Luis Mendía Gorostidi, C.S. Amara Centro.
Lourdes Arregui, C.S. Amara Centro.
Teresa Genover Llimona, C.S. San Rafael.
Francisco Tomas Sesma Aisa, C.S. San Rafael.
Please cite this article as: Agustí A, Fernández-Villar A, Capelastegui A, García-Losa M, Velasco B, Sánchez G. Estudio de la validez de las versiones en catalán, gallego y vasco del cuestionario COPD assessment test y equivalencia con la versión en castellano. Arch Bronconeumol. 2017;53:311–317.