Bronchoalveolar lavage (BAL) analysis has been proposed as an objective technique for confirming asbestos exposure. However, the reliability and diagnostic yield of this procedure has not been studied in Spain. The aim of this study was to assess the usefulness of the analysis of asbestos bodies (AB) in bronchoalveolar lavage (BAL) for the diagnosis of asbestos-related diseases (ARD).
MethodsBAL samples from 72 patients (66 male, mean age 66 years) undergoing bronchoscopy were analyzed. Lung tissue from 23 of these patients was also analyzed. Asbestos exposure was assessed by anamnesis and a review of the patient's medical records. BAL and lung samples were processed and AB count was determined by light microscopy. The accepted threshold value to diagnose asbestos-related diseases was 1 AB/ml BAL or 1000 AB/gr dry tissue.
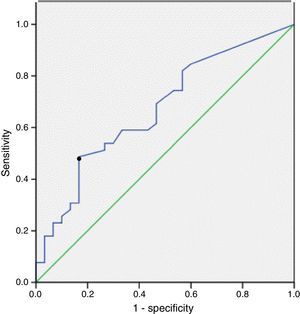
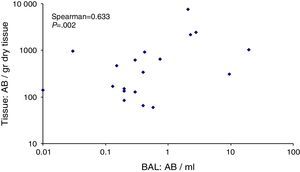
ResultsThirty-nine patients reported exposure to asbestos. Of these, 13 (33%) presented AB values above 1 AB/ml BAL. In the 33 non-exposed patients, 5 (15%) presented AB values above 1 AB/ml BAL. There was a significant difference between the AB levels of exposed and non-exposed patients (P=.006). The ROC curve showed that a value of 0.5 AB/ml BAL achieved the most satisfactory sensitivity, 46%, and a specificity of 83%. The correlation between AB levels in BAL and lung was 0.633 (P=.002).
ConclusionsBAL study provides objective evidence of exposure to asbestos. The good correlation between the AB counts in BAL and lung tissue indicates that both techniques are valid for the analysis of asbestos content.
El análisis del lavado broncoalveolar (LBA) se ha propuesto como técnica objetiva para certificar la exposición a amianto. Sin embargo, la fiabilidad y la productividad de este procedimiento diagnóstico no se han analizado en España. El propósito de este estudio fue evaluar la utilidad del análisis de partículas de amianto (PA) en el LBA para el diagnóstico de enfermedades relacionadas con el amianto (ERA).
MétodosSe analizaron muestras de LBA de 72 pacientes (66 varones, edad media de 66 años) sometidos a broncoscopia. También se analizó el tejido pulmonar de 23 de estos pacientes. La exposición al amianto se evaluó a partir de la anamnesis y la revisión de las historias clínicas de los pacientes. Las muestras de LBA y de tejido pulmonar se procesaron, y la cantidad de PA se determinó mediante microscopia óptica. El valor umbral aceptado para diagnosticar una enfermedad relacionada con el amianto fue de 1 PA/ml de LBA o 1.000 PA/g de tejido seco.
ResultadosTreinta y nueve pacientes refirieron exposición a amianto. En 13 (33%) de estos pacientes, los niveles de PA fueron superiores a 1 PA/ml de LBA. De los 33 pacientes no expuestos, los valores de PA fueron superiores a 1 PA/ml de LBA en 5 casos (15%). La diferencia entre los niveles de PA de los pacientes expuestos y los no expuestos fue significativa (p=0,006). La curva ROC indicó que el nivel de 0,5 PA/ml de LBA era el que alcanzaba mayor sensibilidad (46%), con un 83% de especificidad. El grado de correlación entre los niveles de PA en el LBA y el tejido pulmonar fue de 0,633 (p=0,002).
ConclusionesEl estudio del LBA ofrece una prueba objetiva de la exposición a amianto. La buena correlación observada entre los recuentos de PA en el LBA y en el tejido pulmonar indica la validez de ambas técnicas para analizar el contenido de amianto.
The widespread use of asbestos in industry and its inhalation by workers have prompted the emergence of several asbestos-related diseases (ARD) such as mesothelioma, lung cancer, asbestosis and benign pleural pathologies.1,2 In Spain, given the large quantity of asbestos imported – 2.4 million tons before 19983 – and the fact that its use was not fully prohibited until 2012, the effects of asbestos on the health of the exposed population will continue to be felt in coming years, due above all to the prolonged latency period between onset of exposure and diagnosis of the resulting diseases.
The diagnosis of asbestos-related diseases is based on evidence of exposure and a compatible clinical and radiological profile. Although exposure must always be investigated by anamnesis, in many cases the information that patients provide may be insufficient or confusing. Patients may have been unaware of the presence of asbestos in the workplace, or may have difficulty remembering activities that they performed many years previously. Therefore, in these cases it is important to have access to objective data that prove exposure and permit a reliable diagnosis.4
The most objective proof of exposure to asbestos is its deposition in the lung, which corresponds to the proportion of inhaled asbestos that could not be eliminated. A safe method for determining the extent of this deposition is the analysis of lung samples, for which, obviously, tissue samples are required.5,6 An alternative technique is to analyze asbestos content in the form of fibers, or more commonly, asbestos bodies in bronchoalveolar lavage (BAL). This technique has the advantage of being non-invasive, and in several previous publications has shown a good correlation with the determination of asbestos in lung tissue.7–9
In Spain, the number of recent scientific studies of ARD is limited.6,8–13 Even scarcer are studies that objectively analyze the pulmonary deposition of asbestos.5,6 There is currently only 1 laboratory in Spain that carries out routine analysis of asbestos in biological samples. Laboratory reference values for the Spanish population have been published5 and can be used to establish cut-off points for different pathologies. In the case of BAL, however, no study of this kind is currently available.
The aim of this study was to present an initial assessment of the usefulness of the analysis of asbestos bodies in BAL for diagnosing ARD in the city of Barcelona.
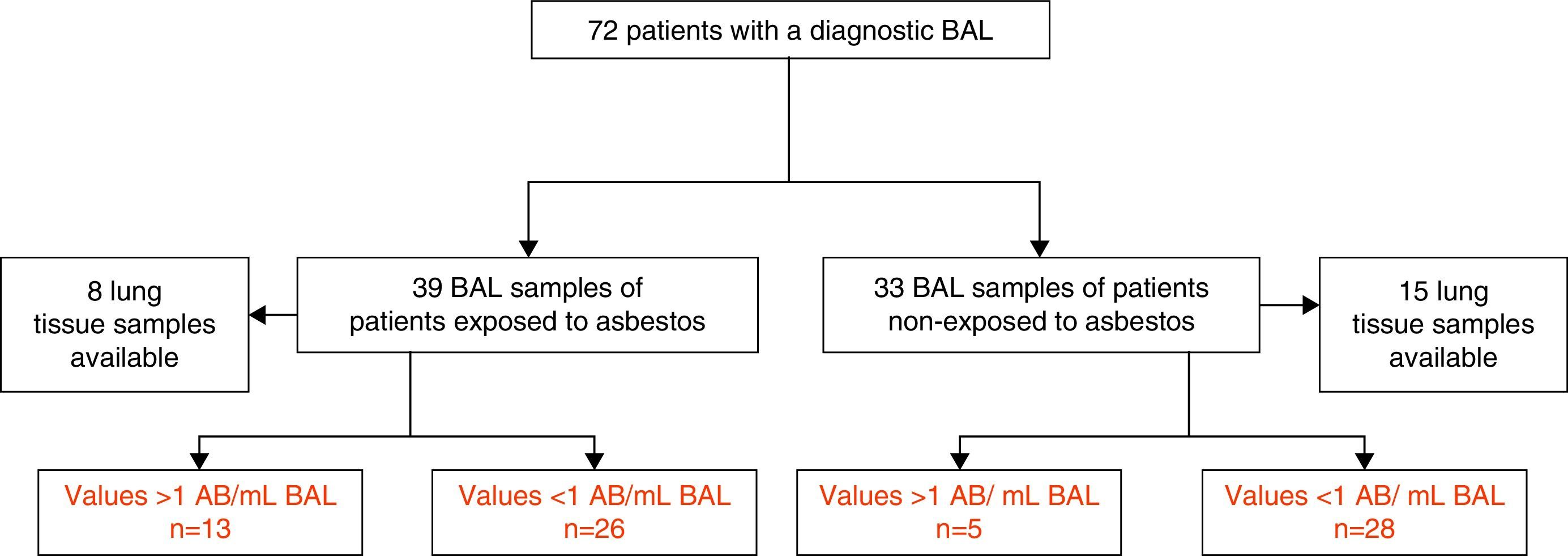
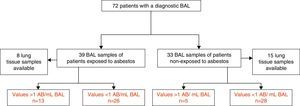
Material and MethodsStudy PopulationThis retrospective-observational study included 39 patients exposed to asbestos who underwent a diagnostic BAL due to suspicion of ARD from 2007 until 2013. Suspicion was based on the patient's report of prior asbestos exposure and/or suggestive chest radiological images. The study also included a second group of 33 patients who had not been exposed to asbestos, in whom BAL was indicated in the diagnostic process. Lung tissue samples were also available from 8 exposed and 15 non-exposed patients (all of whom underwent surgical resection for lung cancer) (Fig. 1).
Fifty-eight patients in the study population were resident in the city of Barcelona, 13 patients in the Barcelona metropolitan area, and 1 in the city of Tarragona. The patient's exposure was determined by anamnesis. Jobs and activities were recorded chronologically and the presence of exposure was established by one of the authors. The study was approved by our hospital's Ethics Committee (PR(AG)20/2007). All patients gave written consent to participate in the study.
Clinical and Radiological DiagnosisAll patients underwent anamnesis, physical examination, chest X-ray, lung CT and blood analysis. The diagnosis of asbestos-related disease was established on the basis of asbestos exposure plus a suggestive clinical and radiological profile.
Lung cancer was diagnosed on the basis of a histological exam performed by a pathologist. The diagnosis of benign pleural ARD was based on radiologic images suggesting the presence of pleural plaques, fibrosis, effusions, and on exclusion of other diseases. Rounded atelectasis was diagnosed on the basis of typical radiological patterns on chest CT. Mesothelioma was diagnosed by the pathologist from the available pleural biopsies using conventional and immunohistochemical techniques. Patients with diffuse interstitial lung disease were evaluated. Asbestosis was diagnosed in patients with a combination of an interstitial lung pattern, asbestos exposure, suggestive pleural alterations or high levels of asbestos bodies in BAL.14
Sample Collection ProtocolBAL samples: Bronchoalveolar lavage was performed at the lingula or right middle lobe of the lung contralateral to the neoplasia. One hundred fifty ml of saline serum were instilled, and at least 10ml of fluid was used for the asbestos study.
Lung tissue: The size of the specimen was 2 cm.3 The region of the lung sampled depended on the tumor location. Samples were fixed in formaldehyde. All lung specimens were examined by a pathologist from our hospital.
Preparation of BAL Lung SamplesTen ml of BAL were obtained from each patient, and centrifuged at 2000×g for 20min, after which 30ml of filtered sodium hypochlorite were added. The mixture was shaken for 1h to facilitate elimination of organic material, and centrifuged at 2000×g for 20min. Sodium hypochlorite was discarded and the sample resuspended in filtered distilled water. The solution obtained was filtered through a 0.45μm pore diameter membrane. The filter was dried in an oven overnight at 37°C and transferred to a microscope slide by means of an acetone vaporizer (JS Holdings 240v/110v) for subsequent study.
Preparation of Tissue Lung SamplesTwo 0.5g fragments of lung tissue that did not contain pleura or vessels were obtained from each specimen. One of these fragments was frozen, lyophilized and weighed to determine the dry tissue weight, in accordance with the international consensus on expressing AB results in terms of grams of dry lung tissue. Once the dry weight was known, the lyophilized sample was discarded, as this technique can alter the concentration and size of asbestos bodies.6
The non-lyophilized tissue section was divided into small fragments and washed for 16h with filtered distilled water. Then, 35ml of previously filtered sodium hypochlorite were added to the sample. The fragments were left in this solution for 4h to facilitate tissue digestion and removal of organic matter.
The sample was centrifuged at 2000×g for 20min. After discarding the supernatant, 30ml of filtered distilled water were added. After another centrifugation and elimination of the supernatant the sample was resuspended in 20ml of filtered distilled water and sonicated for 10″ using an ultrasonic bath (UCI-50, 300W, 50/60Hz, Raypa S.L.).
Twenty ml of a 1:1 ethanol (Milipore Corporation, Germany) chloroform (PanreacQuimicaSau, Spain) mixture were added to the solution and the sample was centrifuged. After centrifugation, the upper layer the resulting density gradient was discarded using a Pasteur pipette and the resulting solution was filtered using a 0.45μm diameter membrane (Millipore Membrane filters HAWP02500). The filter was dried in an oven overnight at 37°C and transferred to a microscope slide by means of an acetone vaporizer (JS Holdings 240v/110v) for subsequent study.
Asbestos Body Counting by Optic MicroscopyThe filters were viewed with an optic microscope (CX21FS2; Olympus Life Science Europe GMBH, Hamburg, Germany) at ×400 magnification. In accordance with the criteria established by the working group of the European Respiratory Society in 1998, asbestos levels exceeding 1 AB/ml BAL or 1000 AB/g dry tissue were considered potentially pathological.7
Statistical AnalysisThe data are expressed as median and range (r). In an initial analysis with the Kolmogorov–Smirnov test, AB values did not follow a normal distribution; hence, the Wilcoxon test was used to determine the differences between groups. The Spearman correlation coefficient was calculated to establish correlations between the parameters analyzed. The consistency of levels of AB in BAL was estimated by evaluating the sensitivity (SE) and specificity (SP) of the method, the positive (PPV) and negative (NPV) predictive values, and the likelihood ratio of a positive (LR+) and negative (LR−) value with 95% confidence intervals (95% CI) using the Wilson method.15 Receiver-operating characteristic (ROC) curves were constructed to determine the most discriminating cut-off value of AB/ml BAL for predicting ARD. The statistical analysis was carried out with Graph Pad software (2002–2005).
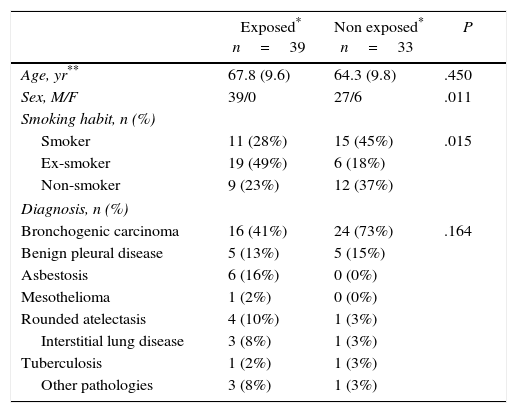
ResultsClinical Characteristics of The Study PopulationThe baseline characteristics of the 72 patients are shown in Table 1. There was a predominance of males and smokers or ex-smokers. Thirty-three patients denied asbestos exposure. Forty patients were diagnosed with carcinoma, 10 with benign pleural disease, 6 with asbestosis, 1 with mesothelioma, 5 with rounded atelectasis, 4 with interstitial lung disease of unidentified origin, 2 with tuberculosis and 4 with other pathologies (bronchiectasis, COPD, aortic aneurysm, esophageal squamous carcinoma). Significant differences between exposed and non-exposed patients with regard to sex and smoking were observed (P=.011 and .015 respectively).
Demographic Characteristics of the Study Population.
| Exposed* n=39 | Non exposed* n=33 | P | |
|---|---|---|---|
| Age, yr** | 67.8 (9.6) | 64.3 (9.8) | .450 |
| Sex, M/F | 39/0 | 27/6 | .011 |
| Smoking habit, n (%) | |||
| Smoker | 11 (28%) | 15 (45%) | .015 |
| Ex-smoker | 19 (49%) | 6 (18%) | |
| Non-smoker | 9 (23%) | 12 (37%) | |
| Diagnosis, n (%) | |||
| Bronchogenic carcinoma | 16 (41%) | 24 (73%) | .164 |
| Benign pleural disease | 5 (13%) | 5 (15%) | |
| Asbestosis | 6 (16%) | 0 (0%) | |
| Mesothelioma | 1 (2%) | 0 (0%) | |
| Rounded atelectasis | 4 (10%) | 1 (3%) | |
| Interstitial lung disease | 3 (8%) | 1 (3%) | |
| Tuberculosis | 1 (2%) | 1 (3%) | |
| Other pathologies | 3 (8%) | 1 (3%) | |
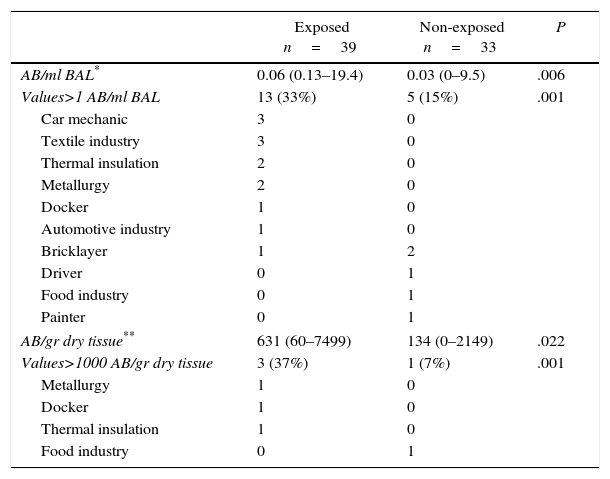
Table 2 shows the levels of AB/ml BAL and AB/g dry lung tissue in exposed and non-exposed patients. Of the 39 exposed patients, 13 (33%) presented values of AB above 1 AB/ml BAL, with a median value of 0.06 (r: 0.13–19.4). In the 33 non-exposed patients, 5 (15%) presented AB values above 1 AB/ml BAL, with a median value of 0.03 (r: 0–9.5). One of these patients lived in Cerdanyola. There was a significant difference between AB levels in exposed and non-exposed patients (P=.006).
Levels of AB in BAL And Lung Tissue in the Study Population.
| Exposed n=39 | Non-exposed n=33 | P | |
|---|---|---|---|
| AB/ml BAL* | 0.06 (0.13–19.4) | 0.03 (0–9.5) | .006 |
| Values>1 AB/ml BAL | 13 (33%) | 5 (15%) | .001 |
| Car mechanic | 3 | 0 | |
| Textile industry | 3 | 0 | |
| Thermal insulation | 2 | 0 | |
| Metallurgy | 2 | 0 | |
| Docker | 1 | 0 | |
| Automotive industry | 1 | 0 | |
| Bricklayer | 1 | 2 | |
| Driver | 0 | 1 | |
| Food industry | 0 | 1 | |
| Painter | 0 | 1 | |
| AB/gr dry tissue** | 631 (60–7499) | 134 (0–2149) | .022 |
| Values>1000 AB/gr dry tissue | 3 (37%) | 1 (7%) | .001 |
| Metallurgy | 1 | 0 | |
| Docker | 1 | 0 | |
| Thermal insulation | 1 | 0 | |
| Food industry | 0 | 1 | |
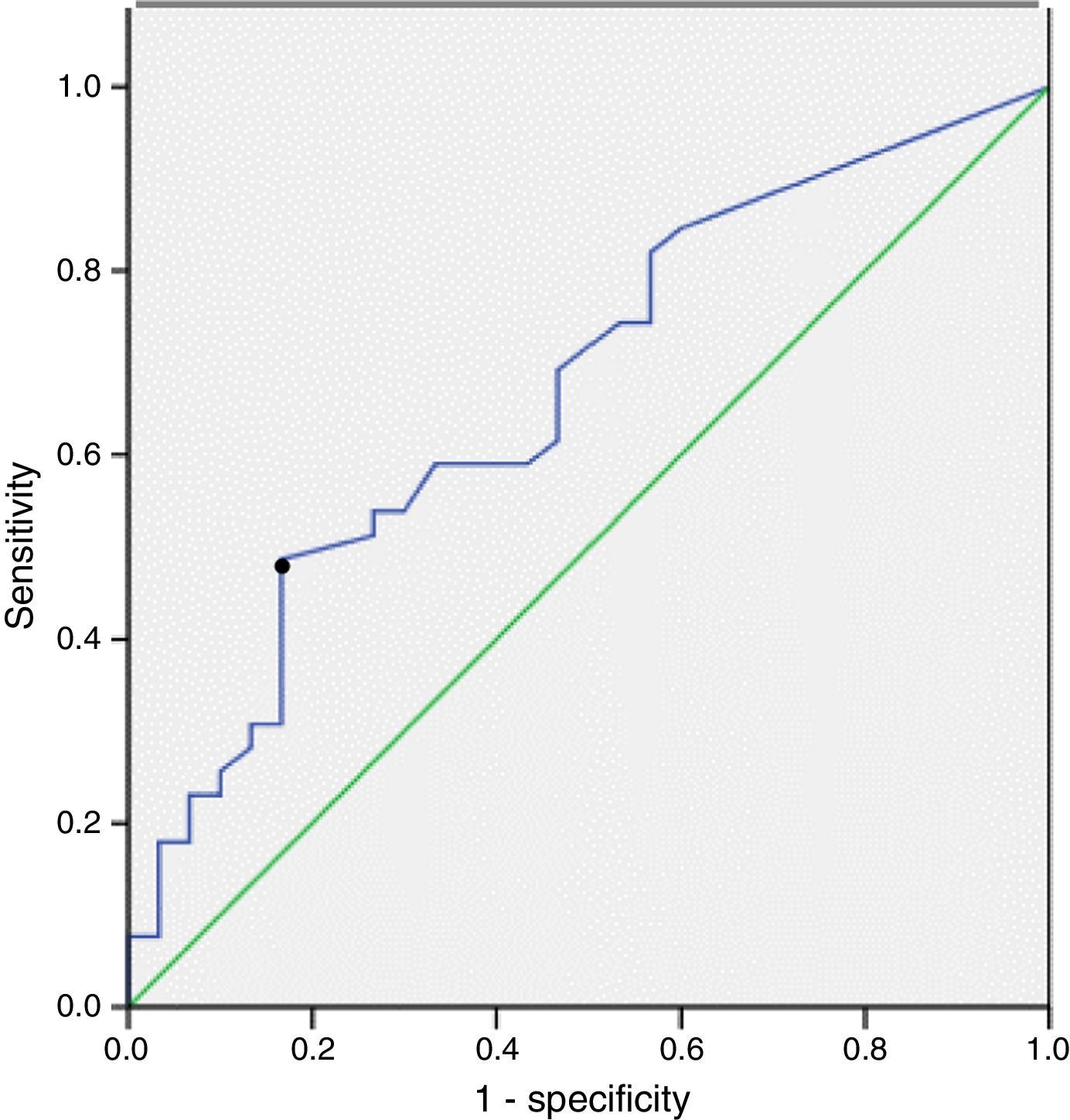
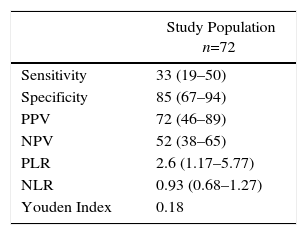
The sensitivity, specificity and positive and negative predictive values of AB in BAL for the diagnosis of ARD, according to exposure determined by work records, are shown in Table 3. AB levels above 1 AB/ml BAL had a sensitivity of 33% (95% CI: 19–50) and a specificity of 85% (95% CI: 67–94) for diagnosing ARD. The ROC curve showed the most relevant cut-off value of AB/ml BAL for predicting ARD. The area under the ROC curve was 0.7. A cut-off point of 0.5 AB/ml BAL achieved the most satisfactory sensitivity: 46% (29–84), with a specificity of 83% (68–96) (Fig. 2).
Sensitivity and Specificity of Quantification of AB in BAL for the Diagnosis of ARD.
| Study Population n=72 | |
|---|---|
| Sensitivity | 33 (19–50) |
| Specificity | 85 (67–94) |
| PPV | 72 (46–89) |
| NPV | 52 (38–65) |
| PLR | 2.6 (1.17–5.77) |
| NLR | 0.93 (0.68–1.27) |
| Youden Index | 0.18 |
Data are expressed as % (95% CI). NLR: negative likelihood ratio; NPV: negative predictive value; PLR, positive likelihood ratio; PPV: positive predictive value.
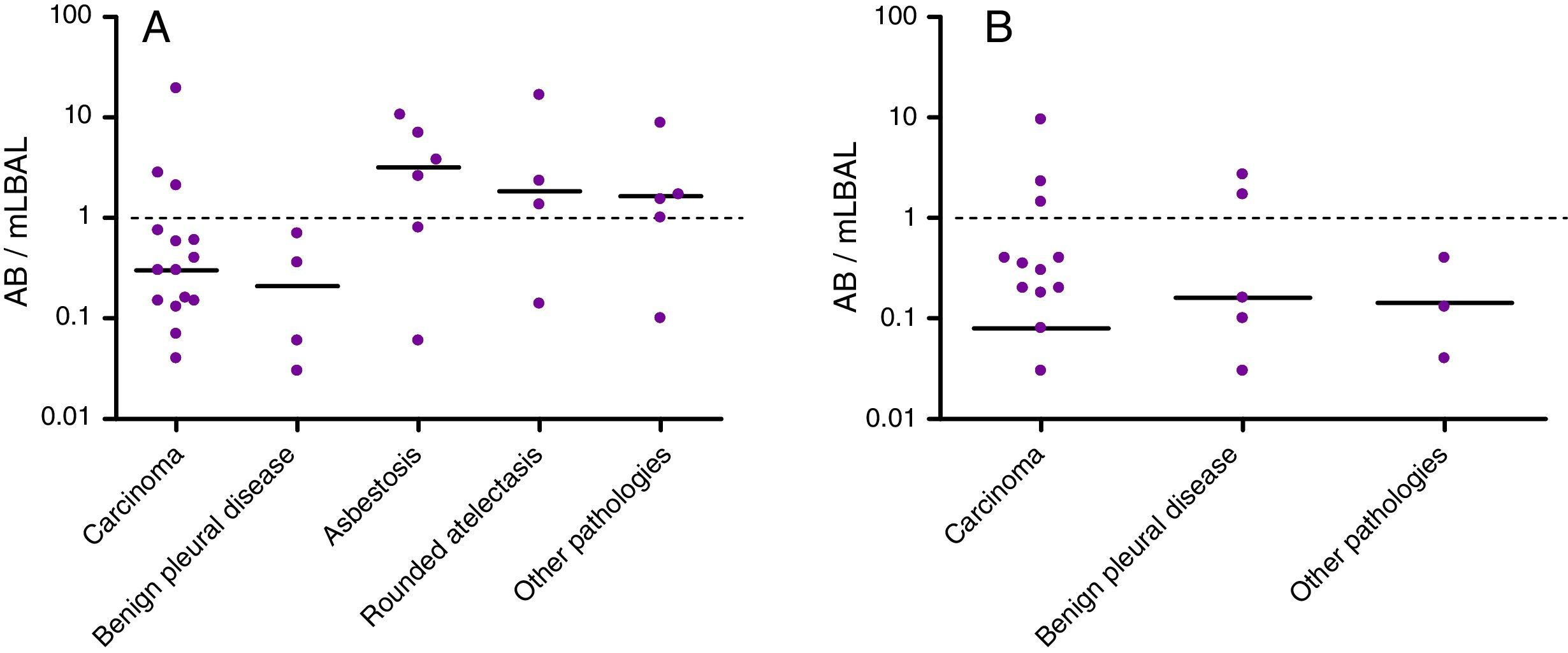
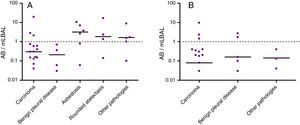
AB levels in BAL in the different pathologies are shown in Fig. 3. Six of the 40 patients with lung cancer (15%) had high levels of AB in BAL: 3 (19%) of the 16 with asbestos exposure and 3 (12%) of the 24 without. Only 2 of the 10 patients with benign pleural asbestos diseases (both non-exposed) showed high AB levels in BAL. Of the 6 patients diagnosed with asbestosis, 4 (all with asbestos exposure) had high AB levels. Three of 5 patients with rounded atelectasis (all with previous asbestos exposure) had high AB levels.
Asbestos Bodies in Lung Tissue and Correlation of AB in BAL and Lung TissueMedians of 631 (r: 60–7499) and 134 (r: 0–2149) AB/g dry tissue were obtained for exposed and non-exposed patients respectively (Table 2). Three (37%) patients in the exposed group and 1 (7%) patient in the non-exposed group presented values above 1000 AB/g.
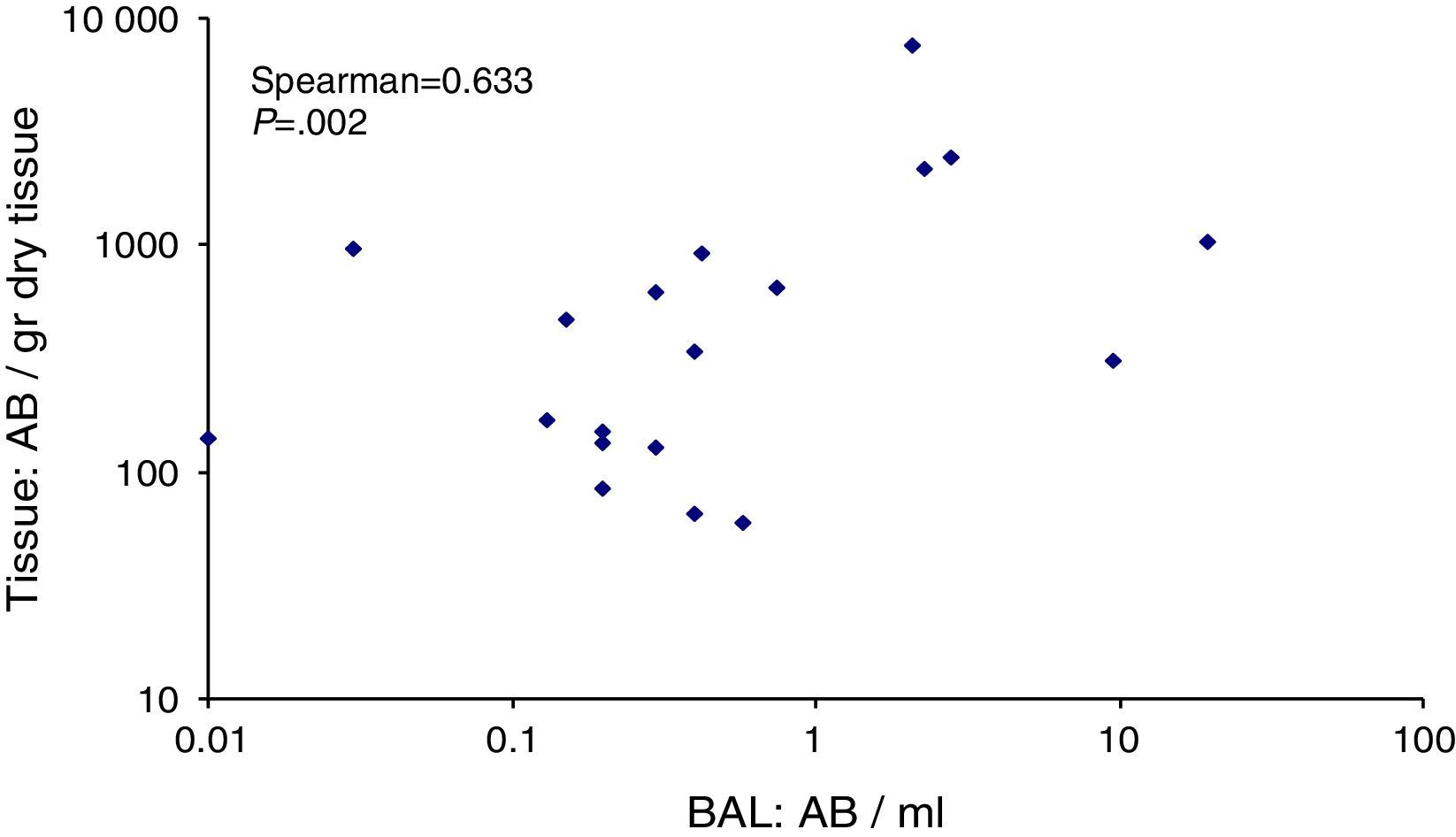
Fig. 4 shows the correlation between levels of AB/ml BAL and AB/g lung tissue, with a Spearman Rank Coefficient of 0.633 (P=.002). None of the 4 patients with lung values above 1000 AB/gr had values below 1 AB/ml BAL, while in 1 patient with lung adenocarcinoma who presented high BAL values this result was not confirmed in tissue.
DiscussionOur study found a good correlation (0.63) between levels of AB in lung and bronchoalveolar lavage. Moreover, no clinically significant discrepancies were observed between these measurements; there was only 1 patient with lung cancer in whom high AB levels in BAL were not confirmed in lung tissue. A value of 0.5 AB/ml BAL showed the best sensitivity (46%, range 29%–84%), with a specificity of 83% (range 68%–96%) for the diagnosis of ARD.
The results of the present study confirm, for the first time in Spain, the diagnostic utility of the quantification of asbestos bodies in BAL. Lung asbestos burden is considered to constitute objective evidence of past exposure to this silicate, and its quantification has been proposed as a useful tool in patients with a suspected asbestos-related disease when a history of exposure is either lacking or inconclusive.7 Because of the difficulty in obtaining lung samples for analysis, certain less invasive diagnostic methods have been investigated. While the sensitivity of sputum analysis is controversial,16–19 BAL has been used by several authors to determine lung asbestos burden.7,20–22 The present study supports this approach, since a significant correlation was observed between the AB counts in BAL and in the lung. Previous studies have found this correlation to be around 0.7.23,24 In our study the correlation was slightly lower, at 0.63, probably due to the characteristics of the patient sample: in previous studies the percentage of non-exposed patients was very low, between 7% and 8%, whereas in our series it was as high as 46%.23,24 The analysis of asbestos in biological samples loses sensitivity in patients with lower AB levels, and results are highly variable owing to the recovery of lavage samples and the counting techniques.7
In our series, AB count in BAL was shown to be a reliable basis for diagnosis of ARD. All patients with high AB counts in lung also had high AB counts in BAL, and high levels in BAL were not confirmed in the lung in only 1 patient. This patient, who had worked as a chauffeur, denied asbestos exposure. The difference in lung and BAL results may have been due to variations in the analyses carried out, which included different lung areas.17 In conclusion, the experience at our laboratory shows that quantification of asbestos bodies in BAL is a good alternative to lung examination when determining asbestos exposure, and offers the advantage of being less invasive for the patient.
The present study highlights the difficulties of ascertaining asbestos exposure in clinical practice. Overall, the diagnostic yield of BAL for determining exposure was moderate, as reflected by a Youden index of 0.18. Although in this study we found the best threshold for AB counts in BAL to be 0.5 in terms of sensitivity and specificity, the accepted international threshold is 1, and we calculated our diagnostic yield accordingly. Only 33% of the patients who reported some degree of asbestos exposure had AB counts above 1/ml. The low AB counts in BAL of exposed patients are surprising, although this phenomenon has already been observed in previous studies.25 The most likely explanation is the difference between exposure and retention. Most imported asbestos is in the form of chrysotile, which is known to have faster clearance than other asbestos fibers. According to this hypothesis, the vast majority of inhaled asbestos would be eliminated, thus explaining the negativity of BAL analyses performed many years later. Indeed, recent data from our group reveal that the vast majority of asbestos fibers retained in the lungs of the Spanish population are amphiboles (unpublished data). Moreover, this limited sensitivity may increase in direct proportion to the latency time,7 suggesting that analyses of lung asbestos burden cannot substitute anamnesis in the initial evaluation of patients. Consequently, a history of exposure must continue to be the cornerstone for diagnosis of asbestos-related diseases. BAL examination, then, remains a practical option for establishing definite exposure in patients in whom the anamnesis is inconclusive.
However, our results also highlight the limitations of anamnesis to reliably ascertain past asbestos exposures. Fifteen per cent of our non-exposed patients had high levels of asbestos bodies in BAL. As other authors25 have noted, unknown exposure, be it occupational, environmental or domestic, may remain unrecognized and this is challenging for physicians diagnosing ARDs. Patients can be unaware of past exposure to asbestos for several reasons, including lack of information on the activities and products existing in the subject's workplace, as well as his or her inability to remember specific information from the distant past. An example of this is the case of a patient in our series who did not report asbestos exposure but had high levels of AB counts in BAL; this patient had lived in Cerdanyola, a town with well-documented, high environmental levels of asbestos due to the vicinity of a fibrocement factory.
Levels of asbestos bodies in BAL varied according to the specific disease. In lung cancer, 15% of patients had high AB counts, suggesting that asbestos exposure should be investigated as a possible risk factor for lung cancer in our setting. The supra-additive effect of asbestos and smoking constitutes a huge health concern in Spain, where the percentage of smokers still remains high. In addition, diagnosing asbestos as the causative factor in lung cancer is particularly difficult in smokers, and the issue often has legal implications.26,27
The relevance of high AB counts in BAL depends on the disease under diagnosis. In our series, in patients with mesothelioma, rounded atelectasis or benign pleural diseases, high AB levels confirmed the diagnosis; while in patients with asbestosis, detection of high levels (66%) supported a specific diagnosis and helped rule out other interstitial diseases.
This study has several limitations. Asbestos exposure could not be ascertained using the job-exposure matrix, and the accuracy of self-reported exposure was probably low. Additionally, since the test results correspond to patients from Barcelona, an industrial city, they cannot be extrapolated to individuals living in rural areas. Finally, patients with mesothelioma were not included, so our results do not apply to this pathology.
In conclusion, our results show that analysis of asbestos bodies in BAL could be a reliable method for determining the lung asbestos burden in the Spanish population. Although its limited sensitivity means that it cannot replace anamnesis as the gold standard for establishing asbestos exposure, the assessment of asbestos bodies in BAL represents a good option in patients with a confusing medical history.
AuthorshipConception and design: MJC and JF; Analysis and interpretation: DAS, VC, JG, MC, LP and ASF; Drafting the manuscript for important intellectual content: MJC and JF.
Conflict of interest statementThe authors declare that they have no conflict of interest.
This Project was supported by the Fis PI07/90478 (Instituto de Salud Carlos III, Madrid), FundacióCatalana de Pneumologia (FUCAP) and Sociedad Española de PatologíaRespiratoria (SEPAR). MJC is supported by the Miguel Servet research program of the Instituto de Salud Carlos III (CP12/03101).
Please cite this article as: Cruz MJ, Curull V, Pijuan L, Álvarez-Simón D, Sánchez-Font A, de Gracia J, et al. Utilidad del lavado broncoalveolar en el diagnóstico de enfermedades relacionadas con el amianto. Arch Bronconeumol. 2017;53:318–323.