Cystic fibrosis (CF) patients present chronic cough as one of the main symptoms, which has an important effect on quality of life and social relations. Our goal was to validate the Spanish version of the Leicester Cough Questionnaire (LCQ) in a group of children and teenagers with CF.
MethodsAfter adapting to Spanish by standardized translation and retro-translation methodology, a sample of 58 stable CF patients from 7 to 18years were recruited from three CF specialized centers in Spain. The questionnaire was administered twice; the second administration (LCQ2) was performed between 2 and 4 weeks later than the first one (LCQ1), in order to analyze the reliability and validity of the Spanish version. To correlate results with health related quality of life (HRQoL) we used the Cystic Fibrosis Questionnaire-Revised (CFQ-R).
ResultsPopulation was composed by 62% male, age 11.7±3.1years and body mass index (BMI) 19±3kg/m2. Total scores from LCQ were: LCQ1 19 (17.75–21) vs LCQ2 19 (16–21) (P=.199). Cronbach's Alpha coefficient was 0.83 for the LCQtotal and for each specific domain was: 0.82 LCQphysical; 0.74 LCQpsychological and 0.62 LCQsocial. Intraclass correlation coefficient was: 0.69 LCQphysical; 0.59 LCQpsychological; 0.45 LCQsocial and 0.71 LCQtotal (good reliability). Relations with CFQ-R showed moderated and significant results: LCQtotalr=0.51 (P<.001) and respiratory symptoms domain r=0.67 (P<.05).
ConclusionThe Spanish version of the Leicester Cough Questionnaire is reliable and valid for children and adolescents with CF and it has good relations with health related quality of life in this population.
Uno de los síntomas destacados de la fibrosis quística (FQ) es la tos crónica, que afecta a la calidad de vida y a las relaciones sociales de los pacientes que la padecen. Nos propusimos analizar la fiabilidad y la validez de la versión en español del Cuestionario de Tos Leicester (Leicester Cough Questionnaire [LCQ]) en niños y adolescentes con FQ.
MétodosTras adaptar el cuestionario al español por el método normalizado de traducción y traducción inversa, 3 centros españoles especializados en FQ reclutaron 58 pacientes de 7 a 18años de edad con FQ estable. El cuestionario se administró en 2 ocasiones (LCQ1 y LCQ2), dejando transcurrir un intervalo 2–4semanas entre ambas administraciones. Para correlacionar los resultados con la calidad de vida (CdVRS) se utilizó la versión revisada del Cuestionario de Fibrosis Quística (CFQ-R).
ResultadosLa población estudiada estuvo compuesta por un 62% de pacientes varones; la media de edad fue de 11,7±3,1años y el promedio de índice de masa corporal de 19±3kg/m2. Las medias de las puntuaciones totales del LCQ fueron las siguientes: 19 (17,75–21) en el LCQ1 frente a 19 (16–21) en el LCQ2 (p=0,199). Se obtuvieron los siguientes coeficientes alfa de Cronbach: LCQtotal 0,83; LCQfísico 0,82; LCQpsicológico 0,74, y LCQsocial 0,62. Los coeficientes de correlación intraclase de las puntuaciones de los dominios LCQfísico, LCQpsicológico, LCQsocial y de la puntuación LCQtotal fueron de 0,69, 0,59, 0,45 y 0,71, respectivamente. Las puntuaciones más bien correlacionadas con el CFQ-R fueron la LCQtotal (r=0,51; p<0,001) y la puntuación del dominio de síntomas respiratorios (r=0,67; p<0,05).
ConclusiónLa versión en español del Cuestionario de Tos Leicester es fiable y válida en niños y adolescentes con FQ; además, en esta población el cuestionario se correlaciona bien con la calidad de vida.
Cystic fibrosis (CF) is a chronic, progressive, genetic disease that affects the exocrine glands, causing multiple organ damage in the respiratory tract, pancreas, liver, sweat glands and reproductive system.1 Pulmonary pathophysiology is characterized by an absent or defective cystic fibrosis transmembrane regulator protein (CFTR) function, which causes abnormal regulation of periciliary liquid volume, decreasing mucociliary clearance and producing mucus plugging and lung obstruction.2 Productive cough is a universal symptom in CF that becomes chronic as the disease progresses.3 Pulmonary exacerbations are defined as an increase in cough and sputum production with loss of appetite and exercise capacity, which has a global impact on school absenteeism.4 As CF progresses, exacerbations occur more frequently and cough becomes a common daily symptom.5
Cough also has a direct influence on survival, and is an important measure of the progress and effective treatment of the disease.6–8 Clinical experience suggests that chronic cough interferes in three dimensions of patients’ lives, namely the physical, the emotional and the social, causing considerable loss of quality of life and affecting social relations.9,10 By monitoring health-related quality of life and the impact of cough, it may be possible to improve treatment efficiency, prolong longevity and decrease the economic impact of the disease.11 Furthermore, cough might be an important factor for predicting and preventing possible exacerbations.12
Health-related quality of life (HRQoL) assessment provides the basis for evaluating the impact of the disease and treatment on activities of daily living not reflected by conventional clinical tests.11,13 Although HRQoL questionnaires correlate with chronic cough, it is poorly represented in these questionnaires,10 so its impact on patients’ lives is not fully characterized, especially in children. Cough is an exacerbation marker which significantly alters HRQoL, and becomes more significant in adolescents with CF.14
NThe Leicester Cough Questionnaire (LCQ) was designed for the objective evaluation of chronic cough and its impact on daily life. It is divided into three domains: physical, psychological and social.15 Murray et al.16 reported good results when it was applied in adults with non-cystic fibrosis bronchiectasis. LCQ has been translated into multiple languages, including Dutch and Chinese,17,18 and is commonly used for assessing the impact of cough in different respiratory diseases. The results are robust, even when cough is acute.19 However, little is known about cough assessment in CF20; these evaluations are generally limited to exacerbations and it is poorly analyzed in children.21
According to recent evidence, the LCQ may be an appropriate tool for assessing cough in a young CF population, but it has not been investigated previously in this specific group.
The aim of this study was to translate and validate the Spanish Version of the Leicester Cough Questionnaire in children and teenagers with cystic fibrosis, in order to provide a new tool for analyzing the impact of cough on this population. The Spanish version of the Leicester Cough Questionnaire could constitute a simple, easily administered instrument for the assessment of cough in young CF patients.
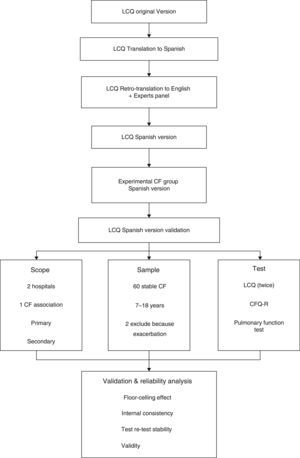
MethodsParticipantsThe study population was recruited in Spain from the Hospital de Sabadell, Corporació Sanitària Universitària Parc Taulí, Sabadell (Barcelona), Hospital Ramón y Cajal and the Asociación Madrileña de Fibrosis Quística in Madrid. All participants were enrolled between May and September 2013. Inclusion criteria were clinically stable CF patients aged 7 to 18 years old, with no exacerbations during the month before study inclusion, who could read and understand the questionnaires. Initially, 60 patients were included, but 2 were excluded due to a respiratory exacerbation during the course of the study (Fig. 1).
The study was approved by the Ethics Committee of the Hospital de Sabadell, Corporació Sanitària Universitària Parc Taulí and Pompeu Fabra University in Barcelona, Spain. Before the study, patients over 14 years of age signed the informed consent form, and for children under 14, consent was signed by parents who agreed to allow their child to participate in the study.
For the purpose of characterization, all patients performed conventional lung function tests22 using a portable spirometer Easyone™ Bluetooth Cradle model 2010BLT (Zurich, Switzerland) in the Madrid hospitals, and Datospir-600 Sibelmed (Barcelona Spain) in Sabadell.
Cough QuestionnaireThe Leicester Cough Questionnaire (LCQ) measures the impact of cough on quality of life.15 It is a self-administered questionnaire composed of 19 items divided into three domains: physical (8 items), psychological (7 items), and social (4 items). Answers are recorded on a 7-point Likert scale. The total score, ranging from 3 to 21, is obtained by adding the domain scores and dividing by three: higher scores represent lower cough impact on quality of life. Each domain may also be analyzed independently.
Quality of LifeThe Cystic Fibrosis Questionnaire-Revised (CFQ-R) consists of a quality of life questionnaire specifically designed for cystic fibrosis patients older than 6 years of age.23 It has been translated to several languages, including Spanish.24 We used three different versions of the questionnaire according to age groups: CFQ-R 6–11 (from 6 to 11 years), CFQ-R 12–13 (from 12 to 13 years) and CFQ-R 14+ (adolescents). The questionnaire consists of self-reported items with different domains, including physical functioning, vitality, health perceptions, respiratory symptoms, treatment burden, role functioning, emotional functioning, and social functioning. Answers are reported on 4-point Likert scale rating frequency, difficulty, or truth, by selecting statements that best describe the patient's situation. Final score is obtained by domains, each domain has a score from 0 to 100; the higher the score, the higher the patient quality of life.
Validation ProcessFor the validation process, we followed the standard forward-backward translation method. First, we translated the original version into Spanish and then a native English official translator retranslated it to English. A panel of experts in respiratory diseases and cough discussed and compared the two versions in order to find the best expressions for better patient understanding. After this process, the preliminary version of the questionnaire was tested by a group of patients (n=8) to obtain their impressions and comments. During the final procedure, some simple modifications were introduced without changing the meaning of the questionnaire. Only two questions were adapted by including a simple common word, in parentheses, similar to the original, in order to facilitate comprehension (Fig. 1).
To validate the Spanish version of the LCQ, the final version was applied twice within a period of 15–30 days to compare both results. During this period, patients were asked not to change their routine management, initiate physical activities, change medication, or participate in any unusual activity that could interfere with their cough status during the validation period. If they did, they were automatically excluded from the study.
Floor or ceiling effects may be considered if more than 15% of the patients achieve the lowest score, meaning higher impact of cough, or the highest possible score, meaning lower impact of cough on quality of life, respectively. Absence of floor or ceiling effects indicates good content validity.25
Statistical AnalysisStatistical analysis was performed with PASW Statistics 18 (SPSS Inc., IBM., USA). Demographic characteristics are represented as median and interquartile range. The Saphiro–Wilk test was used to analyze the distribution of the data. Non-parametric statistics were used because most data were not normally distributed (P<.05).
For reliability, internal consistency and reproducibility were examined. The questionnaire validity (internal consistency) was assessed by Cronbach alpha coefficient and item total correlation coefficients, while the intraclass correlation coefficient (ICC) was used for reliability. Cronbach's alpha coefficient was assumed to be >0.70. Reliability levels were based on the following classification: high reliability, ICC≥0.90; good reliability, ICC≥0.70 and <0.90; acceptable reliability, ICC≥0.40 and <0.70; poor reliability, <0.40. Agreement over time was assessed by constructing a Bland–Altman plot for the LCQ total score by calculating the mean difference between two measurements and the standard deviation (SD) of the difference.26 In this plot, 95% of the differences are expected to be less than 2 SDs.
The minimal important difference (MID) is given as: MID=±1.96×√2×SEM, where 1.96 is derived from the 95% confidence interval (CI).
Measurement error is expressed as a standard error of measurement (SEM), which is calculated as: SD×(√1−ICC), where SD is the standard deviation of values from all participants and ICC is the reliability coefficient.27
The convergent validity was assessed with Spearman's correlation coefficient between the LCQ and the CFQ-R scores. A strong correlation was considered to be over 0.60; a moderate correlation between 0.30 and 0.60; and a low (very low) correlation below 0.30.25
ResultsDemographicsThe final group comprised 58 clinically stable CF patients, 38% girls, mean age 11.7±3.1 years, body mass index (BMI) 19±3kg/m2, with preserved lung function: forced vital capacity (FVC) 2.68±0.93L; forced expiratory volume in the first second (FEV1) 2.25±0.79L; and FEV1/FVC ratio 83.85±6.9%.
Demographics and clinical characteristics are shown in Table 1.
Patient Characteristics.
| Population characteristics (n=58) | |
|---|---|
| Sex: M/F (%) | 62/38 |
| Age (years) | 12 (9–14) |
| Height (m) | 1.47 (1.33–1.58) |
| Weight (kg) | 41.3 (30.2–52.1) |
| BMI (kg/m2) | 18.3 (16.6–21) |
| FVC (L) | 2.45 (2.01–3.37) |
| FVC (%) | 101 (83–114) |
| FEV1 (L) | 2.05 (1.72–2.92) |
| FEV1 (%) | 94 (83–114) |
| FEV1/FVC (%) | 84.4 (78.9–89.6) |
Data are expressed as median and interquartile range. BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in the 1st second; FEV1/FVC: flow/volume ratio; M/F: male/female.
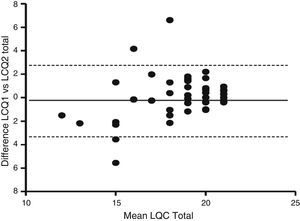
In terms of reproducibility, the Spanish version of the LCQ shows no significant differences in median and interquartile range between both administrations of the questionnaire (Table 2). The results obtained in the three domains and in the total score were similar in both administrations: LCQphysical 6.19 vs 6.50; LCQpsychological 6.43 vs 6.43; LCQsocial 6.75 vs 6.75; and LCQtotal 19 vs 19 [1st vs 2nd administration (P=ns)]. The Bland and Altman analysis showed a higher concordance between total score results when both administrations were compared. A Bland–Altman plot of the LCQ total score is shown in Fig. 2. The validity and reliability results obtained from Cronbach's alpha coefficient and the intraclass correlation coefficient (ICC) are shown in Table 3. Results showed acceptable reliability, ICC≥0.40 and <0.70, except for the ICC of the LCQtotal domain, which was 0.71, showing good reliability. It is important to note that the questionnaire did not present floor effect (0%) in any of the domains.
Differences Between Both Administrations of the Leicester Cough Questionnaire.
| 1st administration | 2nd administration | P-value | |
|---|---|---|---|
| Physical Domain | 6.19 (5.47–6.78) | 6.50 (5.59–6.75) | .504 |
| Psychological Domain | 6.43 (5.43–6.86) | 6.43 (5.71–6.86) | .838 |
| Social Domain | 6.75 (6.00–7.00) | 6.75 (6.25–7.00) | .975 |
| Total | 19 (17.75–21) | 19 (16–21) | .199 |
Data are expressed as median and interquartile range.
Wilcoxon's test *P<.05.
Bland-Altman plot of the LCQ total score and the changes between first and second administration. Solid line represents the mean difference between over two administrations and the dashed lines are the limits of agreement, which represent 2 times the standard deviation of the mean difference.
LCQ Reliability and Validity Analysis.
| Leicester Cough Questionnaire | Floor (%) | Ceiling (%) | Cronbach alpha | ICC (95% CI)a | SEM | MID |
|---|---|---|---|---|---|---|
| Physical Domain | 0 | 36.1 | 0.82 | 0.69 (0.52–0.81) | 0.46 | 1.07 |
| Psychological Domain | 0 | 41.3 | 0.74 | 0.59 (0.38–0.74) | 0.59 | 1.39 |
| Social Domain | 0 | 62.1 | 0.62 | 0.45 (0.20–0.65) | 0.63 | 1.47 |
| Total | 0 | 29.3 | 0.83 | 0.71 (0.54–0.82) | 1.28 | 2.99 |
ICC: intraclass correlation coefficient.
The minimal important difference (MID) for the LCQ was between 1.07 to 3, with standard error measurements (SEM) between 0.46 and 1.28 (Table 3).
Relationship Between the Spanish Version of the LCQ and Health-related of Quality of LifeFor a better understanding of LCQ dimensionality, we compared the dimension results with quality of life according to the Cystic Fibrosis Questionnaire-Revised (CFQ-R). This was done by analyzing all questionnaire domains (physical capacity, role functioning, vitality, emotion functioning, social functioning, body image perceptions, eating, treatment burden, health perceptions, weight perceptions, and respiratory and digestion symptoms). The most significant and interesting correlations are shown in Table 4. Significant correlations with CFQ-R were found for physical capacity, respiratory symptoms and body image domains (P<.001).
Correlations Between the Different Domains of the LCQ, CFQ-R and Lung Function.
| Leicester cough questionnaire domains | ||||
|---|---|---|---|---|
| Physical | Psychological | Social | Total | |
| Respiratory Symptoms domain (CFQ-R Children) | 0.624** | 0.378* | 0.236 | 0.513** |
| Physical Capacity domain (CFQ-R Adolescents) | 0.653* | 0.749** | 0.739** | 0.627* |
| Respiratory Symptoms domain (CFQ-R Adolescents) | 0.700** | 0.734** | 0.684* | 0.670* |
| Body image domain (CFQ-R Adolescents) | 0.577* | 0.575* | 0.599* | 0.637* |
| FVC (L) | 0.427** | 0.601** | 0.462** | 0.557** |
| FEV1 (L) | 0.487** | 0.626** | 0.456** | 0.582** |
Spearman's correlations. CFQ-R Children: Cystic Fibrosis Questionnaire-Revised for children from 6 to 13 years; CFQ-R Adolescents: Cystic Fibrosis Questionnaire-Revised for adolescents over 14 years; FVC: forced vital capacity; FEV1: forced expiratory volume in the 1st second.
The Spanish LCQ version is a valid and reliable questionnaire for evaluating the impact of cough on quality of life in children and adolescents with cystic fibrosis. It is interesting to note that this was the first study in which the LCQ was administered to this population of children; no previous references to this group of patients were found.
The Spanish LCQ version shows similar results to the original version created by Birring et al.15 The original version showed good reliability in all domains and in total score; the Spanish version, however, obtained lower values in both coefficients: Cronbach's alpha coefficient ranged between 0.62 and 0.83 and ICC between 0.45 and 0.71. It should be pointed out that social domain coefficients in the Spanish version were inferior to those in the original version and to those reported in other studies.15,16 These results may be explained by the young age of the sample. Most LCQ-based studies are conducted in adults, because children and adolescents perceive their surroundings in a different way,28 and this affects the final score results, as confirmed by the percentage of young patients (50%) who did not think that having a cough presented a problem. Total scores were repeatable, with intraclass correlation coefficients above 0.7. Thus, repeatability of the LCQ in CF patients was adequate and in accordance with previous results.29
The excellent stability of the Spanish LCQ version reveals lower impact of cough on quality of life in the population studied, most likely due to age and less severe pulmonary disease, suggesting a plausible explanation for the absence of floor effect in the study. Gee et al.30 confirm that severely affected patients tend to produce a greater floor effect, while those with moderate and mild levels generate ceiling effects in most items. A ceiling effect was seen in 15% of our patients in all domains, and could limit the internal validity of the Spanish version. However, these results may be explained by the patients’ mild respiratory severity and young age. Furthermore, sex and age are strong predictors of health-related quality of life, and children reported a lower treatment burden than perceived by their parents.31 Consequently, the lower social domain coefficient obtained in the Spanish version may be due to the patients’ young age (the social impact of cough may still be unimportant in this population, and cough is not an obstacle for their normal activities).
The LCQ is a robust tool for measuring the impact of cough on quality of life, as demonstrated by the strong, significant correlations observed between LCQ and CFQ-R, especially in the physical domain and respiratory symptoms. It is safe to say, then, that the LCQ truly reflects quality of life related to the impact of cough in CF patients.
Subjective tools such as cough visual analog scales, scores, and diaries are widely used to measure the impact of cough. Although the best way of evaluating cough severity is not known, it is likely that a combination of subjective and objective assessments is necessary, and well-validated cough severity assessment tools must be investigated further. It is important to emphasize the utility of validating the Spanish version, confirming its reliability even when completed by minors. This area has not been explored in previous studies.
LimitationsThere were some limitations to the study that are worth mentioning.
First of all, researchers detected difficulties younger patients had some difficulty understanding some of the items. This was solved in the final version by adding a synonym in parentheses next to the misunderstood word.
Another difficulty was that the uneven age distribution that could lead to misinterpretation of some data, since the perception of disease differs between teenagers and children. In addition, our patients had low-grade disease, so results could have been overestimated, increasing the ceiling effect and revealing a better quality of life and lower impact of cough.
The sample was not selected specifically for the validation, and instead was recruited from the ordinary respiratory outpatient population, and this is reflected in the results. If the study group had been composed of moderate-to-severe CF patients, the Spanish version of LCQ could have truly characterized the impact of cough on quality of life. Changing medication regimens in patients could also alter cough status, modifying LCQ results. To diminish this effect, the research team made sure there was no variation in drugs for each patient during the validation process.
Despite these limitations, the aim of the study was to demonstrate the validity of the Spanish LCQ questionnaire version for young CF patients, and not to show if they have more or less cough.
In conclusion, the Spanish version of the Leicester Cough Questionnaire is valid for evaluating the impact of cough on quality of life in children and adolescents with cystic fibrosis. In view of these results, we recommend that this instrument be used regularly to detect any change in quality of life that could appear during the course of the disease.
FundingThis project was partially financed by Proyecto Avanza, TSI-020110-2009-431. Ministerio de Industria, Turismo y Comercio, Spain.
Conflict of InterestsThe authors state no conflict of interests with regard to this manuscript.
The authors would like to thank parents and patients for taking part in this study, and the institutions that allowed them to use their facilities. They are also grateful for the strong support of the Activa team, Norma Trujillo, Lisbeth Hernández, Adrian Rejas, Dr. Jordi Villà and especially, William Lalinde.
Please cite this article as: del Corral T, Percegona J, López N, Valiente A, Garriga M, Seborga M, et al. Validez de la versión en español del Cuestionario de Tos Leicester en niños con fibrosis quística. Arch Bronconeumol. 2016;52:63–69.