To determine the utility of molecular techniques in the diagnosis of resistance and the extent of resistance to first-line drugs in our region.
Material and methodFrom 2004 to 2013, 1889 strains of Mycobacterium tuberculosis complex isolated in Asturias, Spain, were studied using phenotypic (Clinical and Laboratory Standards Institute guidelines) and molecular (INNOLiPA RIF-TB©; GenotypeMDRplus©; GenotypeMDRsl©) sensitivity tests.
Results1759 strains (94.52%) were sensitive to all first-line drugs, and 102 strains (5.48%) showed some resistance: 81 strains (4.35%) were resistant to 1 single drug, 14 (0.75%) were polyresistant, and 7 (0.37%) were multiresistant (resistant to rifampicin and isoniazid). In total, 137 resistances were identified: 60 to isoniazid (3.22%), 7 to rifampicin (0.37%), 9 to pyrazinamide (0.48%), 11 to ethambutol (0.59%), and 50 to streptomycin (2.68%). Of the mutations detected, 75.9% (63/83) correlated with resistance, while 24.09% of mutations detected (20/83) were not associated with resistance; 16 of these involved a silent mutation at codon 514 of the rpoB gene. Between 0% and 90% of strains, depending on the drug under consideration, were resistant even when no gene mutations were detected using marketed systems.
ConclusionsMolecular techniques are very useful, particularly for obtaining rapid results, but these must be confirmed with standard phenotypic sensitivity testing. The rate of resistance in our region is low and multi-drug resistant cases (0.37%) are sporadic.
Conocer la utilidad de las técnicas moleculares para el diagnóstico de resistencias y la situación de las resistencias a fármacos de primera línea en nuestra área geográfica.
Material y métodoDesde 2004 a 2013, 1.889 cepas de Mycobacterium tuberculosis complex aisladas en Asturias, España, fueron estudiadas mediante pruebas de sensibilidad fenotípicas (directrices del Clinical and Laboratory Standards Institute) y moleculares (INNOLiPA RIF-TB©; GenotypeMDRplus©; GenotypeMDRsl©).
ResultadosMil setecientas cincuenta y nueve cepas (94,52%) eran sensibles a todos los fármacos de primera línea y 102 cepas (5,48%) presentaban alguna resistencia: 81 cepas (4,35%) a un solo fármaco, 14 (0,75%) con polirresistencia y 7 (0,37%) multirresistentes (resistencia a rifampicina e isoniacida). En total hubo 137 resistencias a fármacos: 60 a isoniacida (3,22%), 7 a rifampicina (0,37%), 9 a pirazinamida (0,48%), 11 a etambutol (0,59%) y 50 a estreptomicina (2,68%). El 75,9% de las mutaciones detectadas (63/83) se correlacionaron con resistencia; mientras que un 24,09% de las mutaciones detectadas (20/83) no implicaban resistencia, correspondiendo 16 a una mutación silente en el codón 514 del gen rpoB. Entre un 0 y un 90% de cepas, dependiendo del fármaco que se considere, eran resistentes aunque no presentaban mutaciones en los genes incluidos en los sistemas comerciales.
ConclusionesLas técnicas moleculares resultan muy útiles sobre todo por la rapidez en la obtención de resultados, aunque estos deben confirmarse con las pruebas de sensibilidad fenotípicas de referencia. La tasa de resistencias a fármacos en nuestra región es baja y los casos de multirresistencia (0,37%) son esporádicos.
The persistence of tuberculosis (TB) is associated with several factors, some of the most important being socioeconomic problems, increasing rates of HIV coinfection, and growing resistance to antituberculosis drugs.1
The worldwide prevalence of drug-resistant tuberculosis is worrying; the annual incidence of multi-drug resistant tuberculosis (MDR-TB), defined as disease resistant to at least isoniazid and rifampicin,2 is estimated at 500,000 cases, approximately 3.5% of all new cases of TB. Although there are major differences between regions, cases of extremely drug-resistant tuberculosis (XDR-TB), defined as MDR-TB that is also resistant to a fluoroquinolone and at least 1 injectable second-line drug, have been reported in most countries in the world, illustrating the magnitude of the problem.3
Although incidence of TB in Spain continues to fall, reflecting the epidemiological trend observed in other European countries,4 control of the disease is threatened by the appearance of MDR-TB and XDR-TB, and therefore all circulating strains must be studied.
The incidence rate of TB in Asturias in 2013 was 14.98 cases per 100,000 inhabitants,5 just above the Spanish mean of 11.88 cases per 100,000 inhabitants.6
Insight into resistance among Mycobacterium tuberculosis (M. tuberculosis) strains in a specific geographical area can help adjust treatment regimens to the needs of the population, and determine the suitability of existing therapeutic regimens.7
Various methodologies have been used to establish the sensitivity pattern of clinical isolates. The most accurate method and the one that provides fewest false results is the multiple proportions method described by Canetti in 1963,8,9 which is now the most commonly used procedure worldwide.
The delay inherent in standard sensitivity testing has increased interest in molecular techniques, since these provide results in a matter of hours, a critical consideration if the right treatment is to be initiated as soon as possible. The main drawback of molecular testing is that, with the exception of rifampicin, in which 97% of resistances are coded by the rpoB gene, the genetic changes that cause between 20% and 40% of resistances to the remaining first-line drugs are still unknown.10 Another problem is that the high cost of this type of testing may limit its availability in countries with limited economic resources.
Because of the uncertainty surrounding current molecular methods, we must continue to perform phenotypic sensitivity testing, preferably using methodologies meeting internationally accepted quality standards that can be verified using external quality controls to guarantee the reliability of the results. Participation in these quality control programs in Asturias began in 2004.
The main aim of our study was to evaluate the contribution of new molecular techniques to the diagnosis of M. tuberculosis resistance, to compare them with the standard phenotypic method, and to determine the situation of resistance to first-line antituberculosis drugs in Asturias.
Materials and MethodsProspective study including all M. tuberculosis strains from clinical samples isolated in Asturias during the period between January 2004 and December 2013.
Criteria for the inclusion of isolates were as follows: in patients in whom several samples were obtained from the same site, the first M. tuberculosis isolate obtained from each patient was included. In patients in whom samples were obtained from different sites, one M. tuberculosis strain from each site was included.
Clinical and Laboratory Standards Institute11 procedures were followed for sensitivity testing, using Canetti's multiple proportions method in Middlebrook 7H10 as the standard method. The following critical concentrations were analyzed: isoniazid 0.2μg/ml, rifampicin 1μg/ml, ethambutol 5μg/ml and streptomycin 2μg/ml. This method was performed on the 1861 M. tuberculosis strains included in the study, and on WHO quality control strains received periodically during the 10-year study period.
All study strains underwent molecular testing for isoniazid and rifampicin resistance, while ethambutol and streptomycin were only studied for the 102 strains that presented phenotypic resistance to any first-line drug. The commercial tests used for the different targets were rpoB, katG, inhA, gyrA, gyrB, rrs, eis (INNOLiPA RIFTB©; GenotypeMDRplus©; GenotypeMDRsl©).
All information was collected in a specially designed Microsoft Excel database. The recommended statistical tests for comparing a diagnostic technique which yielded binary or dichotomous results (molecular testing: presence/absence of mutation) with the gold standard (phenotypic antibiogram) were used. Contingency tables were constructed to calculate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
ResultsPatients with Microbiological Confirmation of Tuberculosis. Demographic DataBetween January 2004 and December 2013, a total of 1826 cases of microbiological confirmation of TB were recorded in Asturias.
The incidence was higher in men (68.3%) than in women (31.7%), with a ratio of 2.15 men for each woman.
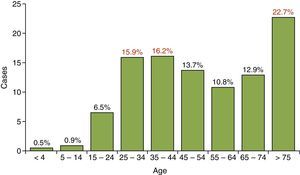
Mean age was 53 years and most cases were detected in the over-75 and 25–44 year age ranges, following the typical bimodal distribution curve. The lowest number of cases of TB was found in the under-15 year age range, with 27 cases (1.4% of the total), as shown in Fig. 1.
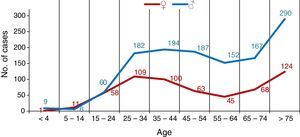
The number of cases of TB was clearly higher in men in practically all age ranges, with the exception of the 5–14 range, which contained more females, and in the 15–24 range, in whom the incidence was the same among the sexes, as shown in Fig. 2.
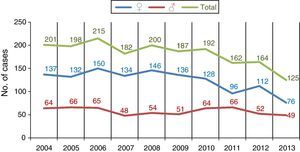
Fig. 3 shows the number of annual cases of tuberculosis in men and women, and reveals a moderate reduction in cases over all age ranges, but mainly among men, and particularly in the 25–44 range.
M. tuberculosis Strains Isolated in Asturias (2004–2013)A patient may have tuberculosis in more than 1 anatomical site, and for this reason the 1826 cases described above presented 1889 strains of M. tuberculosis complex isolated during the study period in the Regional Mycobacteria Reference Unit of Asturias. Mycobacterium bovis (M. bovis) strains isolated in the urine of patients with transitional cell carcinoma bladder receiving BCG instillations were excluded.
The species identified were distributed as follows: 1861 strains (98.51%) of M. tuberculosis hominis; 20 strains (1.05%) of M. bovis bovis; 5 strains (0.26%) of Mycobacterium africanum type I and 3 strains (0.15%) of M. bovis BCG. None of the other species of the M. tuberculosis complex were identified: Mycobacterium canetti, Mycobacterium caprae, Mycobacterium pinnipedi and Mycobacterium microti.
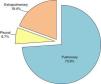
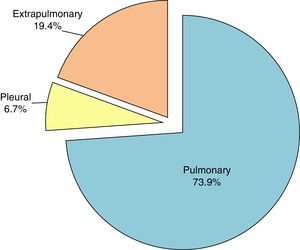
Origin of M. tuberculosis hominis IsolatesThe main site, as expected, was the lung, accounting for 73.9%, as shown in Fig. 4.
The lymph node was the most common site among the cases of extrapulmonary tuberculosis, with 65 cases (3.5%). A more detailed analysis of the source of the study samples is given in Table 1. Samples from purulent exudates, abscesses and ulcers from unspecified sites were classified as “other extrapulmonary samples”, and accounted for 10.8% of the total.
Classification of Study Samples by Site.
| Site of Sample | No. of Samples | % of Total |
|---|---|---|
| Pulmonary | 1375 | 73.9 |
| Sputum | 1196 | |
| Bronchial aspirate | 108 | |
| Bronchoalveolar lavage | 34 | |
| Lung biopsy/FNAB | 23 | |
| Gastric lavage | 14 | |
| Pleura | 124 | 6.7 |
| Pleural fluid | 101 | |
| Pleural biopsy/FNAB | 23 | |
| Extrapulmonary | 362 | 19.4 |
| Lymph nodes | 65 | 3.5 |
| Lymph node biopsy/FNAB | 65 | |
| Genitourinary | 47 | 2.5 |
| Urine | 45 | |
| Epidydimal biopsy | 1 | |
| Endometrial biopsy | 1 | |
| Gastrointestinal | 19 | 1.0 |
| Ascitic fluid | 16 | |
| Omental biopsy | 1 | |
| Colonic biopsy | 1 | |
| Peritoneal nodule | 1 | |
| Bone-joint | 11 | 0.6 |
| Synovial fluid | 4 | |
| Bone biopsy/punch | 3 | |
| Discal FNAB | 2 | |
| Synovial biopsy | 1 | |
| Discal biopsy | 1 | |
| Pericardium | 9 | 0.5 |
| Pericardial fluid | 8 | |
| Pericardial biopsy | 1 | |
| Meninges | 7 | 0.4 |
| CSF | 7 | |
| Other extrapulmonary | 204 | 10.9 |
| Total | 1861 | 100 |
CSF: cerebrospinal fluid; FNAB: fine needle aspiration biopsy.
Of the 1861 strains of M. tuberculosis hominis included in the study, 102 (5.48%) were resistant (initial and acquired) to a first-line drug, as follows: 81 (4.35%) to a single drug, 14 (0.75%) polyresistant strains (resistance to more than 1 drug, except for simultaneous resistance to isoniazid and rifampicin) and 7 (0.37%) MDR-TB (one of these strains was XDR-TB). The 1759 remaining strains (94.52%) were sensitive to all first-line drugs.
Table 2 shows the distribution of the 102 resistant M. tuberculosis hominis isolates, by resistances to each of the first-line drugs.
Distribution of M. tuberculosis Resistance to First-line Drugs in Asturias.
| Resistances | No. | Resistant Strainsa | % of Total |
|---|---|---|---|
| Single resistance | 81 (79.40%) | 4.35 | |
| Isoniazid | 40 | ||
| Rifampicin | 0 | ||
| Ethambutol | 5 | ||
| Streptomycin | 36 | ||
| Pyrazinamide | 0 | ||
| Multiple resistance | 14 | 14 (13.70%) | 0.75 |
| MDR-TB | 6 | 7 (6.90%) | 0.37 |
| XDR-TB | 1 | ||
| Total | 102 | 102 (100%) | 5.48 |
MDR-TB: multi-drug-resistant tuberculosis; XDR-TB: extremely drug-resistant tuberculosis.
Of the 14 polyresistant strains, 13 were resistant to at least isoniazid, 4 to ethambutol, 13 to streptomycin, 7 to pyrazinamide, and none were resistant to rifampicin. Thus, the total number of drug resistances (137) is higher than the number of resistant strains (102).
Phenotypic Sensitivity Testing to First-line DrugsUsing the multiple proportions method as reference, the strains were classified as sensitive or resistant to each drug, according to the different cut-off points previously described, as shown in Table 3.
Nine strains with resistance to pyrazinamide were also detected, but this compound was not included in the study as the multiple proportions method is not a reference method for performing sensitivity testing to this antibiotic.
Molecular Detection of Resistance to First-line DrugsThe frequency of resistance mutations to each first-line drug detected in strains determined as resistant or sensitive using the multiple proportions method is detailed in Table 4. In 20 strains, the presence of mutations did not confer phenotypic resistance, although it should be mentioned that 6 of these had a silent mutation at codon 514 of the rpoB gene, which does not confer resistance to rifampicin.
Molecular Detection of Resistances to First-line Drugs in Resistant Strains and Sensitive Strains According to the Standard Phenotypic Method.
| Standard Method | Resistant Strains | |||
|---|---|---|---|---|
| Genotypic Methods | Mutation | With Mutation | Without Mutation | Total |
| Isoniazid | katG-inhA | 42 | 18 | 60 |
| Rifampicin | rpoB | 7 | 0 | 7 |
| Ethambutol | embB | 4 | 7 | 11 |
| Streptomycin | rrs-eis | 5 | 45 | 50 |
| Standard Method | Sensitive Strains | |||
|---|---|---|---|---|
| Genotypic Methods | Mutation | With Mutation | Without Mutation | Total |
| Isoniazid | katG-inhA | 3 (inhA) | 1798 | 1801 |
| Rifampicin | rpoB | 16 (rpoB514) | 1838 | 1854 |
| Ethambutol | embB | 1 | 90 | 91 |
| Streptomycin | rrs-eis | 0 | 52 | 52 |
Molecular detection of resistance to each of the drugs and their concordance or lack of concordance with the phenotypic methods can be seen in Table 5. The capacity of genotypic methods to detect mutations in our series ranged from 100% in the case of rifampicin to 10% for streptomycin. Specificity of the genotypic methods was very high, with rates of between 98.90% and 100%.
Molecular Detection of Drug Resistances, Concordance or Disconcordance With Phenotypic Sensitivity Methods.
| Drug | Isoniazid | Rifampicin | Ethambutol | Streptomycin | ||||
|---|---|---|---|---|---|---|---|---|
| Mutation | katG-inhA | rpoB | embB | rrs-eis | ||||
| Detection | Present | Absent | Present | Absent | Present | Absent | Present | Absent |
| Resistant strains, n (%) | 42a (70) | 18 (30) | 7 (100) | 0 (0) | 4 (36.36) | 7 (63.63) | 5 (10) | 45 (90) |
| Sensitive strains, n (%) | 3b (0.6) | 1798 (99.83) | 16c (0.6) | 1838 (99.13) | 1 (1.09) | 90 (98.90) | 0 (0) | 52 (100) |
| Sensitivity | 70% | 100% | 36.36% | 10% | ||||
| Specificity | 99.83% | 99.1% | 98.9% | 100% | ||||
| PPV | 93.3% | 69.56% | 80% | 100% | ||||
| NPV | 99% | 100% | 92.78% | 53.6% | ||||
NPV: negative predictive value; PPV: positive predictive value.
The ability of molecular methods to detect resistant strains varies widely, depending on knowledge of the genes involved in resistance to each of the drugs, and the inclusion of these targets in commercial testing systems. In the case of rifampicin, this tool is excellent for detecting and confirming any initial suspicion of multiresistant strains. The analysis of M. tuberculosis strains isolated in Asturias during the 10-year period between 2004 and 2013 revealed that the rate of resistance to first-line drugs is low, and that cases of multiresistance are sporadic.
In our series, as in other publications,6 the population was predominantly male, with a proportion of 2.1 men to each woman. This figure is higher than the Spanish mean of 1.7.
Mean age was 53 years, higher than the Spanish mean of 44 years.5 The lowest number of cases of TB was found in the under-15 year age range (27 cases) representing 1.4% of the overall population.12
As expected, M. tuberculosis hominis was the most commonly identified species, with 1861 strains accounting for 98.5% of the total. The most common sites were the lung (73.9%) and the pleura (6.7%). These percentages are somewhat higher than overall rates in Spain, where 70% of sites are pulmonary and 4.9% are pleural.13
Good tuberculosis control consists of periodically determining the prevalence of M. tuberculosis resistance to antituberculosis drugs. However, the magnitude of resistance to these drugs in Spain is difficult to calculate since very few studies have been published, and those which are available report widely ranging figures.14 The first prospective study on resistance to first-line antituberculosis drugs in a large patient cohort was published in 2014.15 Our overall data on initial and acquired resistance to 1 or more first-line drugs among M. tuberculosis strains revealed rates 5.5% lower than those of the 2014 study (9.2%).
Resistance rates to each of the first-line drugs were lower than the Spanish mean: isoniazid (3.22% vs 6.7%); rifampicin (0.37% vs 1.9%); ethambutol (0.59% vs 1%); streptomycin (2.68% vs 4.4%), and no case of resistance to rifampicin alone was detected. The percentage of MDR-TB strains was also lower than the Spanish mean (0.37% vs 1.2%). Taking into account the direct relationship between the immigrant population and cases of MDR-TB,16 the small immigrant population in Asturias (10.4%)17 compared to other Spanish communities may be a determining factor for these lower rates.
With regard to commercially available molecular testing systems, it must be remembered that not all resistance-related targets are included, nor is the variability among the prevalent mutation types in each geographical area taken into account.10 In our series we found a very high rate of specificity, possibly because the mutation and the corresponding wild-type sequence was analyzed simultaneously.18 In contrast, sensitivity varies widely, depending on the drug analyzed.
Resistance to rifampicin in M. tuberculosis can be explained in 96%–98% of cases by rpoB gene mutations,19 something that has gone a long way to facilitate the development of molecular techniques for the rapid detection of rifampicin resistance in a strain isolated on culture, or even in a clinical sample.
In the case of rifampicin, all cases with phenotypic resistance were also detected using molecular techniques, all 7 of which were multiresistant strains. These techniques, then, help clinicians predict multiple resistances and select the right pharmacological treatment from the start.
However, even in the case of rifampicin, molecular methods cannot replace phenotypic methods, since 16 study strains had a silent TTC/TTT mutation at codon 514 of the rpoB gene that did not confer phenotypic resistance.20 Similar results have also been reported by other study groups using other molecular methods based on detection of mutations in the rpoB gene.21–23
Isoniazid resistance in M. tuberculosis is associated with multiple genes (katG, inhA, aphC, ndh, kasA, furA, ethA), of which the first 2 are the most commonly involved (60%–65% of cases),18 but geographical variations are significant. Of the 1861 strains included in our series, 60 were resistant to isoniazid according to the standard method, and of those, 42 (70%) had mutations in the katG and/or inhA genes. Thirty percent of isoniazid-resistant strains had no alterations in the katG or the inhA genes. The resistance mechanisms in these strains may be caused by other genes involved in isoniazid resistance, not included in commercial tests.10
As for the other first-line drugs, between 50% and 70% of ethambutol-resistant strains contain a mutation in the ethambutol resistance-determining region of the embB gene.24 Currently, commercial tests for the rapid detection of mutations related with ethambutol resistance are based exclusively on the embB306 target.
In our study, 4 (36.36%) of the 11 ethambutol-resistant strains detected by the standard method had embB gene mutations and 7 (63.64%) did not show any of these mutations.
In the case of streptomycin, mutations were found in the rrs and rpsL genes in 55% of streptomycin-resistant isolates.18 Most specific mutations occur in the rpsL gene, and the most common mutation is the one that occurs at codon 43 and is associated with high-level resistance, while mutations in the rrs gene confer intermediate-low resistance25 and occur mainly at codons 530 and 912.26–29 However, there are some strains with low phenotypic resistance that present no mutations in those genes, suggesting that alternative targets, such as the gidB gene (that encodes for a specific 16S rRNA methyltransferase)30 or even efflux pumps, may be involved.31
Commercial tests for detecting streptomycin-resistant mutants have limited results, since mutations of the rpsL gene are not included. In our series, of the 50 strains found to be resistant according to the standard method, 5 (10%) had rrs-eis gene mutations and 45 (90%) did not show any of these mutations.
Molecular techniques provide fast results. However, their sensitivity, while excellent in the case of rifampicin, varies widely depending on the drug under study. These techniques must never replace standard phenotypic sensitivity testing, since a genetic mutation does not always mean phenotypic resistance,20 nor does the absence of genetic mutations totally rule out the possibility of phenotypic resistance. Looking ahead, efforts to identify new mechanisms of M. tuberculosis resistance must be stepped up and currently available methodologies improved with the incorporation of newly identified targets, as in the case of the rpsL gene and streptomycin, the inclusion of the new drugs for the treatment of tuberculosis (fluoroquinolones, linezolid, bedaquiline, delamanid, etc.), and, ideally, the simultaneous detection of all genetic mutations.
ConclusionsMolecular techniques provide fast results, but their sensitivity varies widely, depending on the drug under study, so results must be confirmed with standard phenotypic sensitivity testing. Resistance rates to first-line drugs in Asturias are low, below that of other regions of Spain, and multiresistant cases are sporadic.
Authorship/Contributors- -
Juan José Palacios Gutiérrez: project leader and head of the Unidad de Referencia Regional de Micobacterias del Servicio de Microbiología, HUCA.
- -
Luz María Alba Álvarez: data analysis and manuscript writing.
- -
José María García García: Unidad de Gestión Clínica de Neumología, Hospital San Agustín, Avilés: manuscript correction and project supervision.
- -
María Dolores Pérez Hernández: Servicio de Vigilancia y Alertas Epidemiológicas, Dirección General de Salud Pública, Consejería de Sanidad: contribution and review of demographic data.
- -
Susana Martínez González: review of the references.
- -
SESPA Microbiology Laboratory Network: isolation of strains submitted by the Unidad de Referencia de Micobacterias, HUCA
The authors state that they have no conflict of interests.
We thank Ángela Menéndez González, Macarena Álvarez Fernández and Ángeles Díaz Escalada for the perfection they bring to their daily work.
SESPA Microbiology Laboratory Network includes the Microbiology Services of: Hospital de Jarrio, Hospital Carmen y Severo Ochoa, Hospital San Agustín, Hospital de Cabueñes, Hospital de Jove, Hospital Grande Covián, Hospital Álvarez-Buylla y Hospital Valle del Nalón.
Please cite this article as: Alba Álvarez LM, García García JM, Pérez Hernández MD, Martínez González S, Palacios Gutiérrez JJ, on behalf of the Red de Laboratorios de Microbiología del SESPA. Utilidad de los métodos fenotípicos y genotípicos en el estudio de resistencias de Mycobacterium tuberculosis a fármacos antituberculosos de primera línea. Arch Bronconeumol. 2017;53:192–198.