Video-assisted thoracoscopic surgery has become the technique of choice in the early stages of lung cancer in many centers although there is no evidence that all of the surgical approaches achieve the same long-term survival.
MethodWe carried out a retrospective review of 276 VATS lobectomies performed in our department, analyzing age, sex, comorbidities, current smoker, FEV1 and FCV, surgical approach, TNM and pathological stage, histologic type, neoadjuvant or coadjuvant chemotherapy, relapse and metastasis time, with the main aim of evaluating the survival rate and disease-free time, especially with regard to the two/three versus single port approach.
ResultThe one/four year global survival rate was 88.1% and 67.6% respectively. Bivariate analysis found that the variables associated with survival are comorbidity, histological type, stage, surgical approach and need for chemotherapy. When we independently analyzed the surgical approach, we found a lower survival rate in the single-port group vs the two/three-port group (VATS). Stratifying by tumoral stage (stage I) and by tumor size (T2) survival was significantly lower for patients with single-port group in comparison to VATS approach. In the multivariate analysis, single-port group is associated with a higher risk of death (HR=1.78). In analyzing disease-free survival, differences were found in both cases in favor of two/three port VATS: P=.093 for local relapses and P=.091 for the development of metastasis.
ConclusionsThese results challenge the use of the single port technique in malignant lung pathologies, suggesting the need for clinical trials in order to identify the role this technique may have in lung cancer surgery.
La cirugía toracoscópica videoasistida se ha convertido en la técnica de elección para las intervenciones de cáncer de pulmón en estadio inicial en muchos centros, a pesar de que no se ha probado que la supervivencia a largo plazo sea la misma con todos los abordajes quirúrgicos.
MétodoEfectuamos una revisión retrospectiva de 276 lobectomías practicadas en nuestro servicio mediante cirugía videoasistida, y analizamos la edad, sexo, comorbilidades, tabaquismo, FEV1 y FCV, abordaje quirúrgico, estadios TNM y patológico, tipo histológico, quimioterapia neoadyuvante o coadyuvante y tiempo hasta la recidiva o la detección de metástasis con el objetivo de evaluar la tasa de supervivencia y la duración del periodo sin enfermedad en relación con el abordaje quirúrgico, dos/tres puertos o puerto único, de los pacientes.
ResultadosLas tasas de supervivencia global al cabo de uno y cuatro años fueron del 88,1 y 67,6%, respectivamente. En el análisis bivariante se observó que las variables que se asociaban con la supervivencia eran las comorbilidades, el tipo histológico, el estadio, el abordaje quirúrgico y la necesidad de quimioterapia. Al analizar el abordaje quirúrgico de forma independiente, se observó que la tasa de supervivencia era inferior en el grupo en el que se utilizó la técnica monoportal frente al grupo en el que se utilizaron dos o tres puertos (VATS). Al estratificar a los pacientes según el estadio tumoral (estadio I) y el tamaño del tumor (T2), la supervivencia fue significativamente inferior en los pacientes tratados con el abordaje monoportal, en comparación con la VATS. En el análisis multivariante, el riesgo de muerte fue mayor con la técnica monoportal (HR=1,78). En el análisis del tiempo transcurrido sin enfermedad se observó una tendencia hacia una mayor supervivencia favorable a la VATS con dos/tres puertos, tanto para la recidiva local (p=0,093) como para el desarrollo de metástasis (p=0,091).
ConclusionesEstos resultados cuestionan el uso de la técnica monoportal en las neoplasias malignas de pulmón, lo que sugiere la necesidad de efectuar ensayos clínicos que permitan identificar la función de esta técnica en la cirugía del cáncer de pulmón.
Lung resections using video-assisted thoracoscopic surgery (VATS) for lung cancer have been performed for more than 20 years.1–3 However, it was only after the publication of studies in the early 2000s detailing the extensive experience from a single center with very good results,4 and a multi-center study of 11 surgeons from six centers who underwent certification to assure the uniformity of the procedure,5 that lobectomies using VATS with systematic lymph node dissection became a widespread standard procedure for early-stage non small cell lung carcinoma (NSCLC) in many thoracic surgery departments. The procedure has been shown to decrease postoperative morbidities, shorten length of hospital stay,6 and has a comparable five-year survival rate.7,8
A number of meta-analyses on the safety and effectiveness of VATS lobectomies in the early stages of NSCLC suggest lower relapse rates and lower five-year mortality rates in VATS patients,9,10 although the recent report published by Mathisen11 and other studies12 have questioned these results, raising the need for randomized studies.
At the Thoracic Surgery Department in A Coruña, standard VATS resections were first performed in 2007. After reading about the experiences of the Duke Group using only two ports,13 we gradually began to use this technique in suitablecases.14
In 2010, a number of surgeons in our department began to use uniportal (SP) VATS, based on the initial description from a Chinese research group,15 starting with lower lobectomies and gradually extending the technique to other types of resection surgery.
There are obvious differences between conventional VATS or a SP approach, such as the field of vision, ability to reach all parts of the thoracic cavity, possible bacterial or oncological contamination due to the more frequent insertion of surgical instruments through an unprotected incision, and the need to leave a drainage tube in the incision.
Nevertheless, studies from a number of groups demonstrated the possibility of carrying out different resections using SP, with essentially similar initial results,16–18 and a recent paper from Ng et al.,19 titled “Uniportal VATS – a new era in lung cancer surgery,” discusses the presumed benefits of this technique.
These short-term results may suffice in the case of benign pathologies,20 but in the case of lung cancer, the most important aspect for the patient is their long-term survival.
As a result, five years after starting to use the uniportal technique in our hospital, we decided to conduct a retrospective review of the pulmonary lobectomies performed in our department on patients with malignant disease and at least 1 year of follow up, with the main objective of analyzing their long-term survival.
Patients and MethodThis is a retrospective study that was carried out at the Department of Thoracic Surgery of the University Hospital of A Coruña in Spain (CHUAC). The Institutional Review Board (CEIC) of the Galician Health Service (SERGAS) approved the study, and all patients provided their written informed consent before the procedure. The primary endpoint was survival after the new uniportal VATS pulmonary resection procedure vs the conventional two- or three-port VATS.
We reviewed the computerized clinical records of the 276 patients who underwent pulmonary VATS lobectomy with curative intent with or without preoperative induction chemotherapy due to malignant pathologies at CHUAC between April 2010 and December 2013.
The variables studied for each patient included age, sex, comorbidities, smoking status, forced expiratory volume in 1s (FEV1) and forced vital capacity (FCV), surgical approach, TNM and pathological stage, histological type and neoadjuvant or coadjuvant chemotherapy, relapse and metastasis time, last follow up and moment of death.
Operability criteria determine whether the patient will tolerate general anesthesia and pulmonary resection. Patients with angina pectoris, or those with known underlying cardiovascular disease were risk stratified by additional functional testing. The general functional status of patients was evaluated, and pulmonary function tests with forced vital capacity (FVC), forced expiratory volume in 1s (FEV1) and carbon monoxide diffusion capacity (DLCO) were obtained in each case to evaluate the extent of surgical resection the patient will tolerate.
Mediastinoscopy or endoscopic staging were usually decided when computed tomography (CT) and/or positron emission tomography (PET) showed mediastinal nodes larger than 1cm in the short axis, or any node with a maximum standardized uptake value (SUVmax) greater than 1.5 times background.
Systematic lymph node sampling or dissection is typically performed at the time of surgery to rule out the presence of hilar or mediastinal nodal metastases.
Thoracoscopy was performed at the start of most procedures to assess the extent of the tumor. Patients with early-stage tumors I, II were usually treated using video assisted primary surgical resection, and more advanced cases were usually resected using thoracotomy.
N2 and some lung cancer with hiliar involvement were considered individually for neoadjuvant therapy in a clinical multidisciplinary session
The surgical approach was decided individually by each surgeon. All surgeons had more than 2 years experience in two- or three-port VATS before starting this study.
The technical aspects of conventional and SP VATS have been described in previous papers.14,18 Hilar or mediastinal lymph node dissection was performed on all patients. Lung cancer staging was based on the AJCC 2009 cancer staging reference tools.21
Information on TNM staging and complete resection were obtained from the pathology report.
The mortality rate was obtained from a review of medical records review and through the Civil Register Index Database. There were no follow-up losses.
All patients received a follow-up post-operative thoracic-abdominal scan every three or six months to evaluate relapse and/or metastasis.
The chemotherapy treatment was evaluated individually at a multi-disciplinary clinical session.
Statistical AnalysisA descriptive analysis was made of the variables analyzed, using mean (standard deviation) and median for quantitative variables and frequencies, and percentages for qualitative variables. The base characteristics of the patients were compared according to the type of approach using the Student's t test or the Chi-squared test, as applicable.
The overall survival rate was analyzed using Kaplan–Meier curves, comparing between-group survival using the log-rank test. The hazard ratio values associated with different variables were estimated using univariate Cox regression models. Finally, variables independently associated with the risk of death were identified using a multivariate Cox regression model.
Local relapse-free survival and metastatic relapse-free survival were compared using competing risk survival methodology. Competing risk was death from all causes in both analyses.
Statistical analysis was performed using the program SPSS version 19.0 for Windows. All tests were carried out from a bilateral approach. Values of P<.05 were considered significant.
ResultsGeneral Characteristics of the SeriesDuring the study period, a total of 276 VATS lobectomies were carried out at our hospital (CHUAC). The mean age of the patients was 66.18±9.8 years, 81.5% were male. The majority patients were smokers (40.7%) or ex-smokers (42.1%), and 58.3% had some kind of associated comorbidity. Mean FEV1 was 81.92%±21.49 (range 31.9–140) and mean FVC was 90.3%±18.12. The most frequent histological type was adenocarcinoma (58.7%), followed by squamous cell carcinoma (33.7%), with the majority of cases in stage I(59.8%). A small percentage (15.6%) of patients received neoadjuvant treatment, and 27.5% received adjuvant treatment. Resection was considered complete (R0) in 98.2% of cases.
Seven surgeons participated in the study, and there were no significant differences in relation to survival time P=.613.
The in-hospital mortality rate was 2.2% (9 patients). A total 14 patients (5.1%) required re-operation, while 10 cases (3.6%) needed to be readmitted to hospital. Median follow up was 29.07+15.04 weeks.
Overall SurvivalAfter one year, the overall survival rate was 88.1%, and 67.6% four years after surgery.
Bivariate Analysis of the Variables Generally Associated With Risk of DeathIn the bivariate analysis, we found the variables associated with survival to be presence of comorbidity, histological type, stage, surgical approach, undergoing complete resection, and undergoing neoadjuvant or adjuvant treatment. Patients with comorbidity had a greater risk of death during the follow-up period (HR=2.13), as well as those with other histological types, using adenocarcinoma as a reference (HR=2.16). The TNM stage also increased the risk of death: the higher the stage, the greater the risk of death. The same was found for the overall stage, finding that patients in stage III–IV had a significantly greater probability of dying (HR=2.46) than patients in stage I. With regard to the surgical approach, a higher risk of death was found in the bivariate analysis for patients who had undergone SP, using conventional VATS as a reference. Also, neoadjuvant and adjuvant therapy were associated with a higher mortality rate in the univariate analysis.
Comparison of Conventional VATS vs SP VATSBivariate AnalysisTable 1 shows a comparison of the characteristics of the patients who underwent lobectomy according to whether usual VATS or SP technique was used. The patients in both groups were similar in terms of age, sex, smoking habit, comorbidity, histological classification and stage. Adjuvant and neoadjuvant treatments were similar in both series, and there was no difference in the possibility of carrying out a complete resection. The patients who underwent surgery with conventional VATS had significantly lower levels of FEV1 and FVC. With regard to tumor size, more T3–T4 patients were operated in the SP group (13.1% vs 5.5%), without reaching statistical significance.
Comparative Analysis of Patient Characteristics in VATS or SP Lobectomies.
| Two- and Three-port VATS (n=146) | Uniportal VATS (n=130) | P | |||
|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | ||
| Age, years | 65.9 (10.4) | 65.0 | 66.5 (9.1) | 66.0 | .650 |
| Gender | .995 | ||||
| Male | 119 (81.5%) | 106 (81.5%) | |||
| Female | 27 (18.5%) | 24 (18.5%) | |||
| Smoking habit | .936 | ||||
| Non-smoker | 26 (17.9%) | 21 (16.4%) | |||
| Former smoker | 60 (41.4%) | 55 (43.0%) | |||
| Smoker | 59 (40.7%) | 52 (40.6%) | |||
| Comorbidity | 89 (61.0%) | 72 (55.4%) | .348 | ||
| FEV1 | 78.5 (21.0) | 77.0 | 86.1 (21.6) | 82.9 | .025 |
| FVC | 86.2 (16.9) | 85.0 | 94.7 (18.4) | 93.0 | .001 |
| Histological classification | – | ||||
| Adenocarcinoma | 91 (62.3%) | 71 (54.6%) | |||
| Squamous cell | 49 (33.6%) | 44 (33.8%) | |||
| SCLC | 0 | 1 (0.8%) | |||
| Atypical carcinoid | 0 | 1 (0.8%) | |||
| Others | 6 (4.1%) | 13 (10.0%) | |||
| T stage | .087 | ||||
| T1 | 67 (45.9%) | 53 (40.8%) | |||
| T2 | 71 (48.6%) | 60 (46.2%) | |||
| T3–T4 | 8 (5.5%) | 17 (13.1%) | |||
| N stage | .667 | ||||
| N0 | 109 (74.7%) | 102 (78.5%) | |||
| N1 | 11 (7.5%) | 10 (7.7%) | |||
| N2 | 26 (17.8%) | 18 (13.8%) | |||
| M stage | .999 | ||||
| M0 | 141 (96.6%) | 126 (96.9%) | |||
| M1 | 5 (3.4%) | 4 (3.1%) | |||
| Stage | .737 | ||||
| I | 89 (61.0%) | 76 (58.5%) | |||
| II | 26 (17.8%) | 28 (21.5%) | |||
| III–IV | 31 (21.2%) | 26 (20.0%) | |||
| Neoadjuvant treatment | 21 (14.4%) | 22 (16.9%) | .561 | ||
| Adjuvant treatment | 42 (28.8%) | 34 (26.2%) | .628 | ||
| Complete resection | .999 | ||||
| R0 | 143 (97.9%) | 128 (98.5%) | |||
| R1 | 3 (2.1%) | 2 (1.5%) | |||
Table 1 shows the different events of interest depending on the type of approach. No statistically significant differences were found between groups in terms of the incidence of intraoperative complications, hospital mortality, or the rate of re-admissions or re-operations.
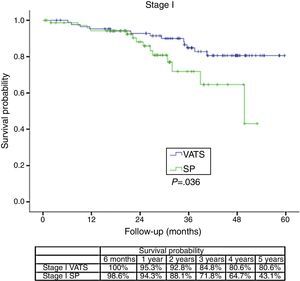
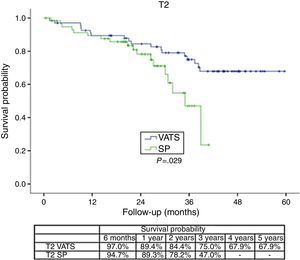
The survival rate during the follow-up period, depending on the type of technique, showed a lower survival rate in SP group vs the VATS group. After stratifying by stage (Figure 1), it can be seen that the survival rate for stage I patients is significantly lower in the case of patients who underwent SP vs conventional VATS. This difference is not observed in patients in stages II and III–IV. Furthermore, after stratifying by tumor size (Figure 2), it can be seen that in T2 patients the survival rate is significantly lower in those who underwent SP VATS vs. conventional VATS. This significance is not observed for the other tumor sizes.
After taking into account the type of surgical approach, age of the patient, stage (using stage I as a reference), comorbidity and the patient's sex, we found the independent predictors of survival to be type of surgical approach, stage, and comorbidity. SP VATS is associated with a higher risk of death (HR=1.78). Stage III–IV is associated with a higher mortality rate vs stage I (HR=2.79). Patients with associated comorbidity also had a higher risk of death (HR=2.51) (Table 2).
Cox Regression Model to Predict Survival Adjusted for Various Covariates.
| Total | Stage I | Stage II | Stage III–IV | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Surgical approach (VATS vs SP) | 1.78 | 1.04–3.04 | 1.31 | 1.01–1.70 | 0.99 | 0.64–1.54 | 1.16 | 0.86–1.56 |
| Age | 1.02 | 0.59–2.57 | 1.02 | 0.98–1.06 | 0.93 | 0.87–0.99 | 1.02 | 0.97–1.08 |
| Gender | 0.85 | 0.43–1.69 | 1.33 | 0.56–3.17 | 1.60 | 0.29–8.75 | 1.17 | 0.02–1.27 |
| Comorbidity | 2.51 | 1.35–4.66 | 2.29 | 0.95–5.54 | 9.78 | 1.58–60.56 | 1.59 | 0.57–4.48 |
| Stage | ||||||||
| I | 1 | – | – | – | ||||
| II | 1.23 | 0.59–2.57 | ||||||
| III–IV | 2.79 | 1.58–4.93 | ||||||
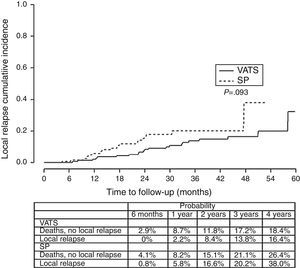
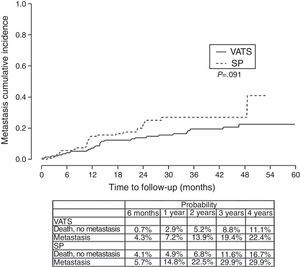
Finally, we analyzed the cumulative probability of relapse (Figure 3) and metastasis (Figure 4) during follow-up after a lobectomy, depending on the different type of technique used. There was a trend towards statistical significance in both cases in favor of conventional VATS (P=.093) for local relapse and for the development of metastasis (P=.091).
DiscussionThe use of minimally invasive surgical techniques is spreading. In thoracic surgery, the changeover from standard thoracotomy to VATS has brought about enormous changes in the post-operative response of patients, as this type of surgery results in less pain, less post-operative complications, shorter hospital stays, possibly less inflammation,22 and in general a better quality of life following surgery.23
This quest for minimally invasive procedures, coupled with instrumental improvements (optics, video equipment, surgical instruments, etc.), seems to have taken the idea of minimum invasion to extremes,20 without any clear indication of whether this is actually beneficial to patients.
We should ask ourselves whether it is worth foregoing one or two small incisions at the expense of losing our field of view, the possibility of separating or accessing the surgical area, and especially whether this approach allows us to achieve at least the same oncological results.
Undoubtedly the main objective of lung cancer surgery is the survival of the patient, and although a number of studies comparing VATS to thoracotomies6–10 have reported similar results in terms of survival, there is no general consensus, and some authors have questioned the wisdom of this approach.12,24 Recently, on the subject of these new techniques, Mathisen stated, “We must not give back the few hard-won percentage points of survival that have been gained over the last four decades,” calling for phase III trials to be carried out in order to obtain evidence on the effectiveness of VATS in terms of its main objective, long-term survival.11
To the best of our knowledge, this is the first comparative study of patients who have undergone surgery using SP, with the main goal of analyzing long-term survival in lung cancer.
In this study, we compared 2 cohorts of patients in which the only selection criteria was the surgeon's interest in using one or another technique, while verifying that both series were homogenous, except for spirometric values (FEV1, FCV) which were significantly lower in conventional VATS patients.
The global one-year survival rate was 88.1%, and four year survival was 67.6%, which is within normal values for lung cancer,21 and is consistent with comorbidity, i.e., patients with a higher surgical risk, and more advanced oncological stage being associated with significantly worse survival rates. Neoadjuvant chemotherapy is given to patients who are doubtful candidates for surgery, with the aim of improving their pre-operative stage in order to offer them the benefits of surgery, while coadjuvant chemotherapy is given to patients in advanced post-operative stages, along with multidisciplinary treatment, which usually determines a worse survival rate. Obviously, the most undifferentiated tumors and those with incomplete resection, can have a significantly worse survival rate.
The fact that SP is also a conditioning factor for survival, and is associated with a higher risk of death (HR=1.78), mainly seems to be justified by the fact that the relapse-free period and metastasis-free period was higher in the conventional VATS group, resulting in worse long-term survival rate in the SP group. The statistical significance of stage I seems to be clearly associated with this being a predominantly surgical stage, where the surgical technique is more closely related with the outcome, while in more advanced stages that usually receive multidisciplinary treatment, the long-term results are more influenced by cancer cells spreading through the ganglia, adjacent structures or metastasis, and the patient's response to chemotherapy or radiotherapy.
When treating early-stages lung cancer, in our opinion and despite the limitations of a retrospective study, the survival difference should be related with the spread of tumor cells during surgery or with persistence of tumor nest at the hiliar, mediastinal o sucarinal level. The lack of protection of the surgical incision or the greater need for surgical manipulation as a result of working with a single incision through which all instruments must be inserted, may favor the spread of cancer cells that may develop locally or metastasize. At the same time, uniportal patients might have been understaged due to inferior ability to harvest nodes using the uniportal technique, thus resulting in inferior survival.
With regard to the significance of the tumor size, it stands to reason that no significance was found for tumors smaller than 3cm, in which, even atypical segmentectomies result in a high level of healing; however, significant differences arise in the case of larger T2 tumors (between 3 and 7cm) which require more complex surgery, more manipulation of the surgical specimen, or even greater difficulty in removing the specimen.
The comparative analysis of survival rates at one, two and four year for SP (90.1%, 80.7% and 61.6% respectively) vs conventional VATS (90.6%, 85.1% and 73.6% respectively) would seem to demonstrate that although both techniques can be useful for pulmonary resection, the significantly worse survival rate after the second year suggests that SP should not be used for lung cancer patients who would have a better survival rate with conventional VATS technique.
The results obtained in our study provide data that contradict the opinion of others who suggest that the SP approach opens up a new era in the treatment of lung cancer,19 based solely on a small number of descriptive short or medium-term studies. In 2014, one of the authors of this article25 suggested a modification to SP, remarking that: “there have been no reported advantages over a two-incision approach to date. In addition, the standard SP approach is associated with these potential disadvantages: the camera competes with the operative instruments, and the chest tube needs to be incorporated within the 4–6cm access incision”.
At this point, we should consider Chang's26 review of two randomized studies comparing stereotactic radiosurgery with surgery in stage I lung cancer. The three-year survival rate showed a statistical significance (P=.037) in favor of radiosurgery (95%), despite the fact that the survival rate for surgery (79%) is very similar to that of our VATS group (80.6%). These studies should make us as surgeons consider whether we should focus more on improving long-term survival rates rather than making small improvements in post-operative stage, were we can never compete with radiosurgery.
The main limitation of this study is its retrospective design, which has hampered the collection of data from the node stations scanned and the number of nodes removed, and surgical wound complications, such as infections, chest wall hernia and possible tumor spread in the surgical incision. However, this has not affected the main objectives of the study, survival, recurrence and metastasis. No selection criteria were applied to the patients other than the surgeon's decision to use one or another technique. In addition, the two series are homogeneous, apart from significantly better spirometric values in the SP group, which lends even further support to the results obtained.
Considering the absence of phase II studies on SP VATS that demonstrate the effectiveness of the technique in lung cancer, the results of our study support the idea that in the treatment of lung cancer, this approach should only be performed as part of a clinical trial, providing the patient with detailed, accurate information about its possible benefits.
AuthorshipJosé M Borro participated in the study design, data interpretation, manuscript preparation, and reviewed the final version after responding to reviewers’ questions.
Francisco Regueiro Mira participated in the clinical review of the patient records, preparation of the study protocol, and review of the manuscript.
Sonia Pértega participated in data analysis and interpretation, and reviewed the conclusions and study limitations.
Manuel Constenla participated in the review of the manuscript.
Salvador Pita Fernández participated in the statistical analysis of the study database, the interpretation of the results of this analysis, and in drawing conclusions and defining study limitations
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: Borro JM, Regueiro F, Pertega S, Constenla M, Pita S. Estudio comparativo de la supervivencia tras procedimientos videotoracoscópicos para la lobectomía del cáncer de pulmón: abordaje por puerto único frente a múltiple. Arch Bronconeumol. 2017;53:199–205.