In the absence of firm recommendations, we analyzed whether unilateral thoracic puncture is sufficient for bilateral pleural effusion (PE), or if the procedure needs to be performed in both sides.
Materials and methodsProspective study of patients seen consecutively for bilateral PE during a period of 3 years and 9 months. All patients underwent simultaneous bilateral thoracocentesis. The standard protocol variables collected in our hospital served as study parameters. Size of PE, presence of chest pain or fever, or accompanying lung abnormalities, different attenuation values on chest computed tomography, presence of loculated pleural fluid, and radiological resolution in a single side were also evaluated.
ResultsA total of 36 patients (19 men; mean age 68.5±16.5 years) were included. The etiology of the effusion was different in each side in only 2 patients (5.6%). In 6/32 cases (18.8%), the biological analysis of the pleural fluid (in terms of transudate/exudate) from both sides did not correspond with the etiological diagnosis of the effusion. Correlation between biochemical parameters analyzed in the fluid from both sides (Pearson's correlation coefficient) ranged between 0.74 (LDH) and 0.998 (NT-proBNP). As different diagnoses in each side were found in only 2 patients, the circumstances in which bilateral diagnostic thoracocentesis would be necessary could not be determined.
ConclusionsSimultaneous bilateral thoracocentesis does not appear to be recommendable. Larger series are needed to establish which factors might suggest the need for simultaneous puncture of both PE.
Ante la ausencia de recomendaciones firmes, se analiza si en un derrame pleural (DP) bilateral es suficiente puncionar un único lado o es necesario hacerlo en ambos.
Material y métodosEstudio prospectivo de los pacientes atendidos de forma consecutiva por un DP bilateral durante 3 años y 9 meses a los que se les hizo una toracocentesis bilateral simultánea. Los parámetros analizados fueron los habituales en el protocolo de nuestra institución. También se valoraron el tamaño del DP, la presencia de dolor torácico o fiebre, o la existencia de anormalidades pulmonares acompañantes, valores de atenuación diferentes en la TC de tórax, presencia de loculaciones pleurales y resolución radiológica en un único lado.
ResultadosSe estudiaron 36 pacientes (19 varones; edad media 68,5±16,5 años). Solamente en 2 enfermos (5,6%) la etiología del derrame fue distinta en ambos lados. En 6/32 casos (18,8%), en cada uno de los lados, el análisis bioquímico del líquido (en términos de trasudado/exudado) no se correspondía con el diagnóstico etiológico del derrame. La correlación entre los parámetros bioquímicos analizados en el líquido de ambos lados (coeficiente de correlación de Pearson) varía entre 0,74 (LDH) y 0,998 (NT-proBNP). Al hallar solamente 2 pacientes con distintos diagnósticos en ambos lados no fue posible evaluar en qué circunstancias puede ser necesario llevar a cabo una toracocentesis diagnóstica bilateral.
ConclusionesNo parece recomendable hacer rutinariamente una toracocentesis bilateral de forma simultánea. Se necesitan series más amplias para establecer qué factores pueden plantear la necesidad de puncionar ambos DP.
Bilateral pleural effusion (BPE) is not an uncommon finding in daily clinical practice1 and it can be caused by a wide variety of diseases.2 To date, no firm recommendations have been made regarding whether BPE can be diagnosed by unilateral thoracocentesis, or if both lungs should be punctured. In a retrospective analysis, Kalomenidis et al. suggest that, in the absence of a specific clinical indication, bilateral diagnostic thoracocentesis (BDT) does not need to be performed as a matter of routine.3 However, this study is limited by the fact that most of the patients (25/27; 92.6%) presented BPE due to heart failure (HF) and coronary artery bypass graft (CABG) surgery, diseases in which the cause of the pleural effusion (PE) is the same in both sides.
In the absence of recommendations, we aimed to analyze whether BDT is necessary in routine clinical practice, or if unilateral puncture is sufficient.
Materials and methodsThis was a prospective study of all BPE patients seen in our department. The protocol was approved by the ethics committee (CEIC 2011/080) and patients gave their informed consent.
BDT was performed in all cases, and a simultaneous blood draw was performed. Samples were sent to the microbiology, pathology and biochemistry laboratories for analysis. The parameters studied in both pleural fluid (PF) and blood were the standard determinations performed in any PE sample in our hospital. A definitive diagnosis was established in all cases.
Biochemical determinations were performed using an ADVIA® 1650 analyzer (SIEMENS Healthcare Diagnostics S.L.). Red cell and nucleated cell counts in PF were measured using an ADVIA® 2120 hematology counter (SIEMENS Healthcare Diagnostics S.L.). A differential count of nucleated cells was performed after cytocentrifugation (450g for 10min) and May Grünwald-Giemsa staining, manually in PF and automatically in blood.
To differentiate transudate from exudate in the analysis of PF, the criteria proposed by Light4 and terminology proposed by Agrawal et al.5,6 were used:
Transudate: Effusions with a pleural fluid to serum (PF/S) protein ratio ≤0.5 and lactate dehydrogenase (LDH) in PF ≤200IU/l (2/3 of the upper limit of normal in serum [300IU/l in our hospital]).
Exudate: Concordant exudates are PEs with PF/S >0.5 and LDH in PF >200IU/l; discordant exudates are effusions classified as exudate with PF/S values >0.5 or LDH in PF >200IU/l, but not both; protein-discordant exudates have PF/S >0.5 with a LDH ≤200IU/L in PF; LDH-discordant exudates have a PF/S ≤0.5 and an LDH in PF >200IU/l.
The etiology of the PE was established from clinical records, physical examination, radiological studies, laboratory findings, and the results of pleural fluid and biopsy analyses. The etiology of pleural transudates was based on clinical and laboratory data, and cytology and negative culture of the PF.2 Patients with suspected HF were only punctured if they had asymmetric BPE, or chest pain or fever.7 Diagnosis of pleural exudate was based on the following criteria: malignant PE (MPE): positive cytology in PF and/or pleural biopsy histology; tuberculous PE: Mycobacterium tuberculosis isolated from pleural fluid or biopsy, or respiratory samples (sputum, bronchial aspirate or bronchoalveolar lavage), or granulomas in pleural biopsy; parapneumonic PE/empyema (PPE): patients with typical signs and symptoms (acute febrile syndrome and pleuritic pain) and pulmonary infiltrates on chest X-ray, with no other explanation for PE; post-injury PE: developing after chest injury with no other explanation; PE of unknown etiology: when none of the diagnostic procedures could identify the etiology of the effusion.
Other data analyzed were PE size (<1/3, >1/3 and <2/3 and >2/3 of the hemithorax), presence of chest pain or fever, or accompanying pulmonary abnormalities, differing attenuation values (Hounsfield units) in the chest X-ray, presence of loculated pleural effusion, and unilateral radiological resolution.
Statistical analysisResults are expressed as mean±standard deviation (SD), or median (interquartile range), depending on whether the data were normally distributed. The Pearson correlation coefficient was used to evaluate the relationship between the biochemical parameters of both effusions.
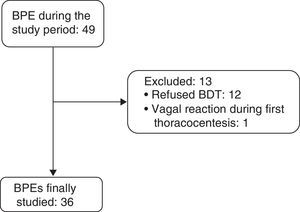
ResultsBetween April 1, 2011 and December 31, 2014, 36 consecutive patients with BPE (19 men and 17 women, mean age 68.5±16.5 years) were included (Fig. 1). Etiologies and origin of the MPEs are shown in Table 1.
Etiology of Bilateral Pleural Effusion and Origin of Malignant Pleural Effusion.
| Etiology | n | Origin of MPE | n |
|---|---|---|---|
| Heart failure | 15 | Lung | 2 |
| Neoplasm | 10 | Breast | 2 |
| Abdominal surgery | 3 | Ovary | 2 |
| Pericarditis | 2 | Lymphoma | 1 |
| Parapneumonic | 1 | Pleura (EHE) | 1 |
| Hypoalbuminemia | 1 | Prostate | 1 |
| Coronary artery bypass graft | 1 | Undetermined etiology | 1 |
| Post-injury | 1 | ||
| Hemothorax | 1 | ||
| Tuberculosis | 1 | ||
| Undetermined etiology | 2 |
EHE: epithelioid hemangioendothelioma; MPE: malignant pleural effusion.
Etiology of right- and left-sided PE was different in 2 patients (5.6%) (hypoalbuminemia/parapneumonic and post-injury/HF) (Table 2, cases 11 and 23). In the remaining patients (34/36; 94.4%), the cause was the same (Table 2). In 6/32 cases (18.8%), the biochemical analysis of the PF (in terms of transudate/exudate) was not as expected from the etiological diagnosis (Table 2). Classification of PF according to Agrawal criteria (transudate, discordant exudate, and concordant exudate) differed between sis in another 6 patients (6/34; 17.6%) (Table 2). Transudate or exudate classification differed in 4 patients (4/34; 11.8%), according to the biochemical characteristics of right- and left-sided PF.
Clinical Characteristics of Study Patients and Biochemical Analysis of Pleural Fluid.
| Sex | Age (years) | PE Size | APA | CP | Fever | Dx R | Dx L | Agrawal Criterion | Agrawal Criterion L | Differences Agrawal Criteria R/L | Cor Dx/Bioch R |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 87 | < | BS | L | No | MPE | MPE | CE | CE | No | Yes |
| F | 60 | < | No | L | No | PER | PER | CE | CE | No | Yes |
| F | 58 | > | No | No | No | MPE | MPE | CE | CE | No | Yes |
| F | 60 | > | L | No | No | MPE | MPE | CE | CE | No | Yes |
| M | 35 | < | No | No | No | UE | UE | CE | CE | No | |
| F | 57 | > | L | R | Yes | HF | HF | DE (LDH) | DE (LDH) | No | No |
| M | 85 | < | R | L | No | HF | HF | T | T | No | Yes |
| F | 50 | > | No | BS | No | MPE | MPE | CE | CE | No | Yes |
| M | 77 | < | No | No | No | HF | HF | DE (LDH) | T | Yes | No |
| M | 77 | < | No | No | No | HF | HF | T | T | No | Yes |
| F | 36 | < | L | BS | Yes | HAB | PPE | T | CE | Yes | Yes |
| M | 84 | < | No | L | No | MPE | MPE | CE | CE | No | Yes |
| M | 60 | < | No | No | No | HF | HF | T | T | No | Yes |
| M | 45 | < | No | No | No | PER | PER | DE (LDH) | DE (LDH) | No | Yes |
| M | 80 | < | No | No | No | HF | HF | DE (LDH) | DE (LDH) | No | No |
| F | 77 | < | BS | No | Yes | UE | UE | DE (PROT) | DE (PROT) | No | |
| F | 81 | < | No | No | No | HF | HF | T | CE | Yes | Yes |
| M | 87 | < | L | No | No | HF | HF | T | T | No | Yes |
| F | 82 | > | No | R | No | CABG | CABG | CE | DE (LDH) | Yes | Yes |
| M | 76 | = | BS | BS | No | MPE | MPE | DE (PROT) | DE (PROT) | No | Yes |
| M | 81 | < | No | No | No | HF | HF | ||||
| F | 76 | < | No | No | Yes | HF | HF | DE (LDH) | DE (LDH) | No | No |
| M | 90 | > | No | R | No | INJ | HF | DE (LDH) | DE (LDH) | No | Yes |
| F | 87 | > | No | No | No | HF | HF | DE (PROT) | DE (PROT) | No | No |
| F | 73 | = | No | L | No | MPE | MPE | CE | CE | No | Yes |
| F | 59 | = | No | No | MPE | MPE | CE | CE | No | Yes | |
| F | 90 | = | No | R | No | HF | HF | T | T | No | Yes |
| M | 67 | = | No | No | MPE | MPE | CE | CE | No | Yes | |
| F | 78 | = | No | L | No | HF | HF | T | T | No | Yes |
| M | 74 | = | No | No | Yes | ABS | ABS | CE | CE | No | Yes |
| M | 74 | = | No | No | Yes | ABS | ABS | CE | DE (LDH) | Yes | Yes |
| M | 29 | < | No | BS | No | TB | TB | CE | CE | No | Yes |
| F | 72 | > | BS | MPE | MPE | ||||||
| M | 60 | < | No | ABS | ABS | T | DE (LDH) | Yes | No | ||
| F | 55 | = | No | No | Yes | HF | HF | T | T | No | Yes |
| M | 47 | > | No | R | No | HEM | HEM | CE | CE | No | Yes |
| Cor Dx/Bioch L | PF/S R | PF/S L | LDH R (IU/L) | LDH L (IU/L) | CEA R (ng/dL) | CEA L (ng/dL) | BNP R (pg/mL) | BNP L (pg/mL) | ADA R (U/L) | ADA L (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | 0.69 | 0.72 | 1.157 | 1.044 | 3.8 | 19.3 | 1.897 | 1.932 | 30 | 32 |
| Yes | 0.65 | 0.71 | 577 | 433 | 0.6 | 0.3 | 511 | 383 | 24 | 20 |
| Yes | 0.74 | 0.67 | 411 | 513 | 0.3 | 0.4 | 46 | 88 | 21 | 25 |
| Yes | 0.74 | 0.72 | 401 | 350 | 1.537 | 1.294 | 362 | 284 | 22 | 21 |
| 0.66 | 0.66 | 961 | 838 | 1.3 | 0.6 | 22 | 44 | 29 | 31 | |
| No | 0.33 | 0.34 | 355 | 476 | 1 | 0.7 | 5.833 | 4.670 | 16 | 17 |
| Yes | 0.31 | 0.33 | 70 | 80 | 0.6 | 0.5 | 39.567 | 39.963 | 9 | 9 |
| Yes | 0.55 | 0.61 | 353 | 293 | 1.2 | 2 | 1.071 | 958 | 16 | 17 |
| Yes | 0.46 | 0.39 | 280 | 179 | 1.5 | 0.9 | 2.147 | 988 | 26 | 18 |
| Yes | 0.32 | 0.32 | 137 | 139 | 1 | 1.2 | 565 | 569 | 14 | 13 |
| Yes | 0.27 | 0.52 | 151 | 813 | 0.5 | 0.5 | 166 | 481 | 13 | 36 |
| Yes | 0.58 | 0.77 | 372 | 446 | 1.6 | 1.8 | 1.342 | 1.510 | 33 | 27 |
| Yes | 0.33 | 0.32 | 156 | 134 | 1.6 | 1.7 | 4.139 | 3.636 | 13 | 12 |
| Yes | 0.41 | 0.34 | 291 | 264 | 0.9 | 0.5 | 7.507 | 8.654 | 17 | 15 |
| No | 0.45 | 0.38 | 294 | 250 | 0.8 | 0.4 | 4.803 | 4.594 | 26 | 24 |
| 0.60 | 0.57 | 118 | 110 | 0.8 | 0.8 | 638 | 641 | 16 | 16 | |
| No | 0.34 | 0.54 | 158 | 220 | 1.2 | 1.6 | 9.113 | 10.549 | 14 | 22 |
| Yes | 0.38 | 0.31 | 106 | 177 | 0.2 | 0.2 | 17.281 | 19.102 | 18 | 19 |
| Yes | 0.70 | 0.44 | 471 | 283 | 0.2 | 0.2 | 2.768 | 2.053 | 26 | 19 |
| Yes | 0.54 | 0.54 | 188 | 147 | 0.2 | 0.2 | 1.585 | 1.587 | 26 | 21 |
| 1 | 0.9 | 10.321 | 10.727 | 14 | 15 | |||||
| No | 0.47 | 0.44 | 306 | 339 | 1.3 | 1.1 | 1.024 | 1.091 | 23 | 21 |
| No | 0.45 | 0.43 | 316 | 203 | 2 | 1.8 | 5.763 | 5.599 | 75 | 25 |
| No | 0.63 | 0.64 | 113 | 97 | 1.8 | 1.8 | 3.494 | 3.258 | 13 | 15 |
| Yes | 0.75 | 0.77 | 247 | 228 | 0.7 | 0.6 | 214 | 227 | 26 | 24 |
| Yes | 0.78 | 0.78 | 260 | 324 | 1 | 1.3 | 1.662 | 1.720 | 31 | 36 |
| Yes | 0.31 | 0.38 | 137 | 152 | 0.5 | 0.5 | 19.282 | 17.520 | 7 | 8 |
| Yes | 0.69 | 0.66 | 366 | 290 | 561 | 476 | 539 | 594 | 12 | 11 |
| Yes | 0.35 | 0.30 | 83 | 82 | 1.7 | 1.2 | 3.895 | 4.530 | 11 | 9.8 |
| Yes | 0.61 | 0.52 | 1.070 | 380 | 0.8 | 0.7 | 547 | 590 | 37 | 25 |
| Yes | 0.62 | 0.44 | 378 | 380 | 1.3 | 0.7 | 335 | 590 | 42 | 25 |
| Yes | 0.58 | 0.65 | 300 | 326 | 2.9 | 2.8 | 186 | 176 | 121 | 94 |
| 93 | 91 | 15 | 58 | 250 | 230 | 8 | 12 | |||
| Yes | 0.27 | 0.40 | 67 | 203 | 1.3 | 1.6 | 2.337 | 2.312 | 3 | 6 |
| Yes | 0.41 | 0.39 | 130 | 146 | 1.2 | 0.7 | 41.376 | 42.560 | 3 | 2 |
| Yes | 0.76 | 0.76 | 463 | 429 | 0.6 | 0.4 | 286 | 233 | 30 | 28 |
ABS: pleural effusion due to abdominal surgery; ADA: adenosine deaminase in pleural fluid; APA: accompanying pulmonary abnormality; BNP: N-terminal propeptide brain natriuretic peptide in pleural fluid; BS: both sides; CABG: pleural effusion due to coronary artery bypass graft; CE: concordant exudate; CEA: carcinoembryonic antigen in pleural fluid; Cor Dx/Bioch: correspondence between final diagnosis and biochemical diagnosis of pleural effusion; CP: chest pain; DE (LDH): discordant exudate (LDH); DE (PROT): discordant exudate (protein); Dx: diagnosis; F: female; HAB: hypoalbuminemia; HEM: hemothorax; HF: heart failure; INJ: injury; L: left; LDH: lactate dehydrogenase; M: male; MPE: malignant pleural effusion; PE: pleural effusion; PER: pericarditis; PF: pleural fluid; PPE: parapneumonic pleural effusion; PROT: proteins; PF/S: pleural fluid/serum ratio; R: right; T: transudate; TB: tuberculous pleural effusion; UE: pleural effusion of undetermined etiology; =: pleural effusion of similar size; > greater in the right side; < smaller in the right side.
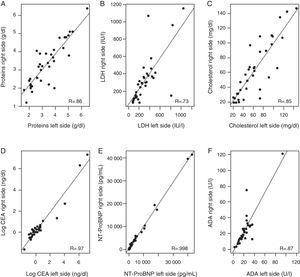
Correlation between biochemical parameters analyzed in right- and left-sided PF [total protein, LDH, cholesterol, N-terminal propeptide of brain natriuretic peptide (NT-ProBNP), carcinoembryonic antigen (CA), and adenosine deaminase (ADA)] (Fig. 2) ranged from a Pearson correlation coefficient of 0.74 for LDH, and an almost perfect correlation (R=0.998) for NT-ProBNP. The lowest correlation was observed for LDH, because levels of this enzyme differed notably in right- and left-sided PF in 2 patients (1 with hypoalbuminemia and PPE in 1 side and 1 patient who had undergone abdominal surgery).
Correlation between levels of various biochemical parameters in the pleural fluid in both sides. (A) Total proteins; (B) lactate dehydrogenase (LDH); (C) cholesterol; (D) carcinoembryonic antigen (CEA); logarithmic; (E) N-terminal propeptide of brain natriuretic peptide (NT-ProBNP); (F) adenosine deaminase (ADA).
Right- and left-sided PE size was similar in 9 patients (25%); greater in the right side in 9 (25%) (4 MPE, 2 HF, 1 injury, 1 CABG, and 1 hemothorax); and greater in the left side in 18 (50%) (9 HF, 2 MPE, 2 pericarditis, 2 PE with undetermined etiology, and 1 each of the following: abdominal surgery, PPE, and tuberculous PE) (Table 2).
The chest radiographs of all patients were examined to determine the existence of associated pulmonary abnormalities. One patient (3.1%, HF) showed an abnormality in the right side, 4 patients in the left side (12.5%; 2 HF, 1 MPE, and 1 PPE), and 4 patients in both sides (12.5%, 3 MPE, and 1 PE of underdetermined etiology). No abnormalities were visualized in 27 (75%) patients.
No chest pain was found in 17/32 patients (53.1%), 5 patients (15.6%; 2 HF, 2 CABG, and 1 hemothorax) complained of pain in the right side, 6 patients had pain in the left side (18.7%, 3 MPE, 2 HF, and 1 pericarditis), and 4 in both sides (12.5%; 2 MPE, 1 tuberculous PE, and 1 in a patient with PE caused by hypoalbuminemia and pneumonia).
Only 7/34 of the patients (20.6%) had fever, of which 3 were cases of HF, 2 abdominal surgery, 1 PPE, and 1 PE of undetermined etiology (Table 2).
Etiology of left- and right-sided PE differed in only 2 of our patients, so it was impossible to evaluate statistically the need for performing BDT based on clinical characteristics, biochemical features of the PF, or radiological findings (attenuation values on chest CT expressed in Hounsfield units determined in 15 patients, or radiological resolution of single-side PE, determined in all cases).
DiscussionThe results of our study confirm that right- and left-sided PE etiology does not differ in most BPE patients (94.6%), so systematic, simultaneous performance of a BDT does not appear to be beneficial. As BPE with different etiology in either side occurs in so few cases (Contarini's syndrome), we could not specify the circumstances under which double puncture would be advisable. A reasonable approach would be to reserve this procedure for cases when the clinical picture suggests 2 possibly different etiologies, or if clinical progress is atypical. This practice may, in some cases, cast doubt on the interpretation of PE as transudate or exudate, due to variability in biochemical results from right- and left-sided PF, even though this does not imply a different etiology in both sides.
In a retrospective study, Kalomenidis et al. found that examining both right- and left-sided PF does not provide any more information than studying unilateral fluid.3 A unilateral puncture will reduce the number of thoracentesis-related complications, and eliminating unnecessary medical procedures will reduce costs. However, Kalomenidis’ findings may be explained by the fact that 25/27 of their patients had HF or CABG, and a common disease mechanism can be expected to cause accumulation of PF of similar characteristics in both sides. Thus, BDT should probably be indicated in an unknown number of patients with BPE. Our series, which includes a wider range of BPE etiologies, appears to confirm these results, since right- and left-sided PE etiology differed in very few (5.6%) BPE patients.
However, if a BDT is not performed in certain cases, essential clinical data may be missed and significant diagnostic errors may occur – for example, if localized pleural disease is concurrent with another condition that can affect both sides. In this situation, observed in 2 cases in our series, the PF of the locally affected side (PPE and post-injury in our series) may be different from the PF in the contralateral hemithorax, indicating differences in the systemic disease (hypoalbuminemia and HF, in our case).
Moreover, while the etiology of the BPE may be the same, the characteristics of the PF in each side may differ significantly; this may be the case in PPE and MPE. In PPE, if the patient presents bilateral pneumonia with BPE, the pleural inflammatory response may differ in each side. In this case, it would be very important to determine as soon as possible the existence of complicated PPE/empyema, so that it can be treated more intensively than the uncomplicated contralateral PE.8 Similarly, in malignant BPE, different pH, glucose and LDH values may be observed in either side, if the extent of malignant pleural infiltration differs.9 Since low pH and glucose levels are indicative of a greater tumor load and can predict failure of pleurodesis and shorter survival, it is important to bear this possible discrepancy in mind when planning optimal patient management. While none of these situations occurred in our series (our only PPE was unilateral, and in all MPEs PF had similar biochemical values), the possibility remains. A good approach would be to determine which factors would predict a benefit for BDT. After analyzing the reasons for performing BDT in the 12 cases of Contarini's syndrome published to date, experts recommend this approach in certain situations: presence of atypical clinical findings (fever or chest pain associated with decompensated HF), unilateral pulmonary parenchymal involvement, PE of significantly different sizes, markedly different PE attenuation values on CT (Hounsfield units), unilateral PE resolution, etc.10–17 (Table 3). However, in our series none of these factors was associated with BPE with different etiology in both sides, probably due to the small number of patients with a double diagnosis for BPE. It seems reasonable to perform BDT in cases where it is unclear if the BPE has the same etiology (based on both clinical and radiological criteria), or if the patient's clinical progress is poor after starting treatment.
Etiology of Bilateral Pleural Effusion of Different Causes (Contarini's Syndrome) Appearing in the Literature, and Reason for Performing Bilateral Diagnostic Thoracocentesis.
| Author | Reference | n | Diagnosis of Right PE | Diagnosis of Left PE | Reason for BDT |
|---|---|---|---|---|---|
| Porcel et al. | 10 | 5 | Subphrenic abscess Pericardial disease Complicated PPE Empyema HF | Fluid overload Simple PPE Post-radiation pleuritis HF Complicated PPE | Chest X-ray images showing different pulmonary and pleural features in both sides |
| Jarcho | 11 | 1 | HF | Empyema | BDT not performed. Retrospective interpretation of findings from autopsy performed 350 years previously |
| Kutty y Varkey | 12 | 1 | Malignant | Empyema | Fever |
| Lawton et al. | 13 | 1 | Malignant | Malignant chylothorax | Differences in attenuation values (Hounsfield units) in chest CT between both Pes |
| Fred | 14 | 1 | Chylothorax | Malignant | Asynchronous development of BPE |
| Brannen y Berman | 15 | 1 | Chylothorax | Malignant | Previous publication21 with similar diagnosis |
| Dixit et al. | 16 | 1 | Empyema | Tuberculosis | Immunosuppression (HIV infection) |
| Khan et al. | 17 | 1 | Malignant | Chylothorax | Rapid increase in size of right PE |
BDT: bilateral diagnostic thoracocentesis; BPE: bilateral pleural effusion; CT: computed tomography; HF: heart failure; HIV: human immunodeficiency virus; PE: pleural effusion; PPE: parapneumonic pleural effusion.
The biochemical parameters analyzed in the PF from both sides correlated well (range 0.74–0.998), although this may have been overestimated, as the values for some parameters varied widely (CA and N-TProBNP). Marked differences were observed between the values from each side in only 3 patients (for LDH and ADA), 2 of whom were the patients with a double diagnosis for PE. In the patient with PE due to injury with raised ADA, the presence of other diseases which might also present with high values of this enzyme was ruled out, such as tuberculosis, other infectious diseases, or lymphoma. Other differences emerged when deciding if the PF was transudate or exudate, depending on the side under consideration (4/34; 11.8%). Using the Agrawal criteria (concordant exudate, discordant exudate, and transudate)5 gave a percentage of 17.6% (6/34 in each side). Errors in both the diagnosis and management of these patients may then occur if useful tests are overlooked or unnecessary tests are performed in the attempt to identify the source of the PE. However, this may be due more to the lack of consistency in the parameters used to differentiate transudate from exudate than to the BDT, as we found that the final PE diagnosis did not correspond with the biochemical analysis of PF in 6/32 cases in each side (18.8%; practically all HF). In this respect, it should be remembered that 15–30% of transudates are wrongly classified as exudates.2
The major limitation of our study is the small number of patients included, which made it impossible to establish the determining factors for performing a BDT. However, no prospective studies in cohorts larger than our series are available in the literature, in view of the difficulty in finding this type of patient, and the unlikelihood of them agreeing, after an ethical explanation, to undergo BDT. Another limitation is that not all patients undergo a chest CT to evaluate the factors influencing the decision to perform a BDT. This test is not systematically performed; it is done only in cases in which the diagnosis is doubtful.
In conclusion, in almost 95% of cases, the etiology of BPE is the same in both sides, so routine, simultaneous BDT does not appear to be recommendable. This procedure should be reserved for cases in which the clinical data suggest different etiologies, or when the clinical picture is atypical. Larger series are needed to establish which factors might indicate the need for bilateral puncture. Correlation between the biochemical parameters of the PF analyzed in both sides is good, and discrepancies in classifying a PE as transudate or exudate due to different biochemical results in PF in each side do not point to a different etiology for the BPE in each side.
AuthorshipL. Ferreiro. Author and editor. Conception and design. Final approval of the manuscript.
M.E. San José. Coauthor. Editor. Responsible for biochemical analyses. Final approval of the manuscript.
F. Gude. Coauthor. Data analysis and interpretation. Final approval of the manuscript.
A. Lama. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
J. Suárez-Antelo. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
A. Golpe. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
M.E. Toubes. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
F.J. González-Barcala. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
J.M. Álvarez-Dobaño. Coauthor. Data collection. Critical review of the article. Final approval of the manuscript.
L. Valdés. Redactor and author. Conception and design. Final approval of the manuscript.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Ferreiro L, San José ME, Gude F, Lama A, Suárez-Antelo J, Golpe A, et al. Derrame pleural bilateral: ¿toracocentesis uni o bilateral? Estudio prospectivo. Arch Bronconeumol. 2016;52:189–195.