Asthma clinics (AC) are hospital outpatient services specializing in the management of asthma. In this study, we analyzed the impact of these clinics on asthma management and their cost effectiveness in comparison with standard outpatient services.
MethodsA case–crossover study in which all new patients seen in the AC of Lugo in 2012 were included. The case period was defined as one year following the first visit to the AC; the control period was defined as the preceding year. We calculated changes in clinical quality indicators for asthma management, and estimated the incremental cost-effectiveness ratio (ICER) for each additional patient treated and for each quality-adjusted life year (QALY).
ResultsThe number of patients (n=83, mean age 49±15.2years; 60.2% women) managed in the AC increased from 41% to 86%. The asthma control test score increased from 18.7±4.6 to 22.6±2.3 (P<.05) and FEV1 increased from 81.4±17.5% to 84.4±16.6% (P<.05). The number of exacerbations, hospitalizations and visits for accident and emergency fell by 75%. The number of patients given combination LABA+ICS therapy fell from 79.5% to 41%. The use of other drug therapies increased as the following: anticholinergics, from 3.6% to 16.9%; ICS in monotherapy, from 3.6% to 45.8%; and omalizumab, from 0% to 6%. ICERs per patient managed and per QALY gained were €1399 and €6876, respectively (social perspective).
ConclusionsTreatment in ACs is cost effective and beneficial in asthma management.
Las unidades monográficas de asma (UMA) son consultas hospitalarias implementadas para lograr una mejoría clínica de los pacientes. Este estudio analiza su impacto sobre el control del asma y su coste-efectividad en comparación con las consultas ordinarias.
MétodosEstudio de casos cruzados que incluyó a todos los pacientes que fueron atendidos por primera vez en la UMA de Lugo durante 2012. Se definió el «periodo-caso» como los 365días que siguieron a la primera visita en la UMA, y el «periodo-control» como los 365días que la antecedieron. Se calcularon los cambios en indicadores clínicos relevantes para el control del asma y se estimó la relación de coste-efectividad incremental (RCEI) por cada paciente adicional que fue controlado y por cada año de vida ajustado por calidad (AVAC).
ResultadosEl porcentaje de pacientes (n=83, edad media 49±15,2años; 60,2% mujeres) controlados aumentó del 41 al 86%. El resultado del test de control del asma mejoró desde 18,7±4,6 hasta 22,6±2,3 (p<0,05) y el FEV1 se elevó desde 81,4%±17,5 hasta 84,4%±16,6 (p<0,05). Las exacerbaciones, hospitalizaciones y visitas a urgencias disminuyeron un 75, un 78 y un 75%, respectivamente. La utilización de combinaciones CI/LABA decreció del 79,5% al 41%. El uso de otros fármacos aumentó: anticolinérgicos del 3,6 al 16,9%, CI en monoterapia del 3,6 al 45,8%, y omalizumab del 0 al 6%. Las RCEI por paciente controlado y por AVAC ganado fueron de 1.399 y 6.876€, respectivamente (perspectiva social).
ConclusionesLa atención en una UMA es coste-efectiva y tiene un impacto beneficioso sobre el control del asma.
According to a report published by the World Health Organization, 300 million people worldwide suffered from asthma in 2004, and its prevalence continues to rise.1 In Spain, around 5% of the adult population are affected by the disease.2 Despite therapeutic advances and the implementation of clinical practice guidelines,3,4 the disease is poorly controlled in between 50% and 70% of asthma sufferers.5,6 A recent Spanish report found that in 3.9% of asthmatics, the disease is severe and poorly controlled.7 The AsmaCost8 study estimated the annual cost per asthma patient at €2635, with 50% of this due to severe cases.9 The 2004 COAX study estimated the mean cost of a moderate asthma exacerbation at €1230, and a severe exacerbation at €3543.10
These figures show the need for strategies aimed at improving follow-up of asthma patients and their interaction with specialist physicians, coupled with a more personalized approach in which individual patient needs are recognized and pharmacological or behavioral (education and follow-up of therapeutic compliance) interventions undertaken.11 All these strategies should be spearheaded by asthma clinics (AC). These outpatient services, staffed by multidisciplinary teams and headed by an expert in the disease would not only provide a comprehensive service, but also play a major role in optimizing economic resources. Nevertheless, previous studies have failed to assess the cost effectiveness of a specialized asthma clinic for both patients and the health service.
Materials and MethodsDesignRetrospective, observational, crossover study with the primary objective of evaluating the cost effectiveness of managing individuals diagnosed with asthma seen for the first time in 2012 in the Hospital Universitario Lucus Augusti (HULA) AC in Lugo, Spain.
The HULA AC (which was opened in 2010) comprises 2 consulting areas (medical and nursing) staffed by asthma specialists. The multidisciplinary team also includes staff from the allergy and immunology clinic. The AC performs all the tests and studies required for the diagnosis and follow-up of asthma patients (spirometry, plethysmography, oscillometry, fractional exhaled nitric oxide, methacholine and mannitol challenge, sputum cell count, etc.) Patients with exacerbations are treated during normal working hours in the pulmonology day clinic, where biological therapies are administered. All patients are taught how to manage their disease and their therapy, and are given a written treatment schedule. The AC is also involved in clinical research and helps train residents and pulmonologists from all over Spain. Asthmatics are referred to the AC from primary care or other specialist centers at the discretion of their attending physician, and not exclusively due to poor disease control. According to the head of the AC, it is equally important to care for patients with well-controlled asthma (workload permitting) for 2 main reasons: to ensure correct diagnosis and to taper treatment once the disease is under control. Due to either lack of training or poor diagnostic methods, diagnosis and treatment follow-up are rarely guaranteed outside the setting of an AC.
For the purpose of the study, data were sourced from IANUS, the Galician Health Service electronic medical record database, which includes details such as date of medical consultation, tests performed, and changes in therapy. “Case-period” was defined as the 365 days following the first consultation at the AC, and “control-period” was defined as the preceding 365 days. The clinical status and cost of care for each patient during the year preceding and following the first evaluation in the AC were compared.
The study was approved by the Clinical Research Ethics Committee (CREC) of Galicia (code 2014/180).
PatientsThe clinical records of all patients (mostly poorly controlled) aged≥18 years, previously diagnosed with asthma, and seen in the AC from 1 January 2012 to 1 January 2013, were included in the study. In some patients referred to the AC with a diagnosis of asthma, the disease was later ruled out. These patients were not followed up.
Study ProtocolDetails of the clinical and functional status and analytical tests performed on all study patients were extracted from IANUS, together with their use of healthcare resources (visits to primary care physicians and specialists due to exacerbations, days of hospital stay, emergency care, asthma medication and diagnostic tests performed). Other data included loss of labor productivity days due to visits to the doctor, the emergency room, and hospitalization, and the cost of traveling to the AC. All study data were uploaded to an electronic case report form (CRF) for subsequent statistical analysis.
VariablesStudy variables and their definitions [diagnosis of asthma, definition of control, exacerbation, cost, and quality-adjusted life year (QALY)] are summarized in Table 1. Cost variables included direct medical costs met by the Galician regional health service (SERGAS) relating to use of resources (asthma medication, visits to the doctor, diagnostic tests and hospitalization), the cost to the patient of traveling to the AC (direct non-medical cost), and the cost to society in terms of loss of labor productivity (indirect cost) for both the year preceding and following referral to the AC. The reference year for costs was 2012. Being a 1-year follow-up study, no discount rate was applied. Table 2 shows the itemized cost of resources and the data source.
Definition of Study Variables.
| Variable | Definition |
|---|---|
| Previous asthma diagnosis | Patient diagnosed with and treated for asthma before referral to the HULA AC. This diagnosis could be ruled out by the AC specialist on the basis of a methacholine challenge test. |
| Asthma | Clinical diagnosis of asthma and positive results on at least 1 GEMA-approved objective diagnostic test (positive bronchodilator test, positive bronchial challenge test, FENO>30ppb).4 |
| Controlled asthma | Minimally symptomatic or asymptomatic patient (ACT≥20), with normal or near-normal lung function (FEV1>70% predicted), and no exacerbations. |
| Asthma exacerbation12 | |
| Severe | Events that require administration of systemic steroids or an increase in the existing dose for at least 3 days (if the 2 courses of steroids are separated by at least 1 week, the exacerbation is considered separate). Visit to the emergency room and hospitalization with administration of steroids. |
| Moderate | Deterioration in the patient's symptoms beyond the usual range of day-to-day asthma variations, requiring a change in the patient's usual medication, but which is not severe. Visits to the emergency room not requiring administration of steroids. |
| Costs | |
| Direct medical costs | Costs met directly by the regional healthcare authority. |
| Drugs | Difference between the total cost of asthma drugs during the month preceding evaluation by the AC asthma specialist (based on the cost shown in the electronic prescription) and the total cost of the drugs administered during the last month of the 12-month period following evaluation in the AC. The difference was extrapolated to 12 months to account for changes in the price of the drugs over the study year. |
| Visits to the doctor | The cost of each visit (primary care physician, day hospital, specialist, or emergency room) taken from the official regional gazette (DOGA) for 2012.13 |
| Hospital admission | The cost of each day of hospitalization was taken from the official regional gazette (DOGA). |
| Diagnostic tests | The cost of functional tests (spirometry with or without bronchodilator, FENO and methacholine challenge), analytical tests (complete blood count, IgE, blood gases), skin prick test panels and radiological studies were taken from the DOGA. |
| Direct non-medical costs | Direct non-medical costs are those met directly by the patient or their family members in connection with the disease or obtaining medical care (e.g., travel expenses met by the patient, services provided by professional carers, alternative therapies). In this study, only travel expenses met by the patient are included. |
| Travel expenses | Average cost to the patient of traveling from their place of residence to the AC or health center to receive treatment for asthma (emergencies or scheduled consultations). A cost of €0.19/km was established, based on the allowance set by the Ministry of the Economy for the use of personal vehicles.14 This per/km rate was multiplied by the mileage shown in the IANUS (Galician Health Service electronic medical record database). |
| Indirect costs | Economic assessment of the losses incurred by society due to the disease. In general terms, this includes loss of labor productivity by the patient or their family members, if these latter are in charge of caring for the patient. In this study, only a rough estimate of loss of labor productivity by the patient is included. |
| Loss of labor productivity | Mean cost of absenteeism among asthmatic employees due to visits to the doctor and hospitalization. The cost per day of absenteeism was calculated using the human capital model, under which the loss of daily productivity is measured in monetary terms on the basis of the mean wage earned in 1 day.15 In this study, gross average wages in the region of Galicia, bracketed by age and sex, were taken form the Wage Structure Survey conducted by the Spanish Institute of Statistics.16 |
| Total costs | The sum of direct medical costs, direct non-medical costs and indirect costs. |
| Quality-adjusted life years (QALY) | QALYs measure health-related quality of life (HRQL) on a scale of 0 to 1, where 1 represents 1 year with the best possible health status. They are calculated on the basis of the patient's self-perceived health status. In this study, QALYs in pre- and post-AC referral patients were based on the estimations made by Doz et al.,6 who attributed 0.89 and 0.69 QALYs to controlled and uncontrolled patients, respectively. Following this, the corresponding QALYs were adjusted for decrease in HRQL for each day with exacerbation over a 12-month period. This was based on NICE estimations that each day with exacerbation not requiring hospitalization costs the patient 0.002 QALYs, and each day with exacerbation requiring hospitalization costs 0.001 QALYs.17,18 |
AC, asthma center; ACT, asthma control test; DOGA, official gazette of Galicia; FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1s; GEMA, Spanish asthma management guidelines; HULA, Hospital Universitario Lucus Augusti, Lugo; HRQL, health-related quality of life; IANUS, electronic medical record system used by SERGAS; IgE, immunoglobulin E; QALY, quality-adjusted life years; SERGAS, Galician regional health service.
Itemized Costs and Sources.
| Cost (€) | Source | |
|---|---|---|
| Diagnostic tests (cost per test) | ||
| Spirometry | 40 | a |
| Post-BD spirometry | 80 | a |
| FENO | 50 | a |
| Methacholine | 200 | a |
| Chest X-ray | 18 | b |
| CT scan | 120 | b |
| Blood count | 3 | b |
| IgE | 6 | b |
| Prick test | 15 | b |
| Drugs (monthly cost of treatment) | ||
| Salmeterol “200/100”/“500/100”/“1000/100” | Fluticasone 45/61/83 | c |
| Budesonide “160/4,5”/“160/9”/“320/9”/“620/18”/“640/18”/“160/4.5” | Formoterol 16/51/32/128/64/16 | c |
| Beclometasone “100/6”/“200/10”/“400/24” | Formoterol 12.5/25/51 | c |
| Fluticasone “200”/“1000”/“2000” | 16/52/104 | c |
| Budesonide “200”/“400”/“800” | 5/8/19 | c |
| Indacaterol 150 | 50 | c |
| Tiotropium 18 | 49 | c |
| Glycopyrronium | 47 | c |
| Montelukast 10 | 21 | c |
| Mometasone “200”/“400” | 15/26 | c |
| Nasal Mometasone “200”/“200” | 16 | c |
| Fluticasone inhaler “55”/“110”/“200” | 3.25/6.5/13 | c |
| Deflazacort “6”/“30” | 9/45 | c |
| Omalizumab “150”/“300”/“450”/“900”/“1200” | 355/710/1065/1420/2840 | c |
| Medical services (cost per service) | ||
| Primary care consultation (follow-up consultation) | 34.45 | d |
| Emergency room | 359.79 | d |
| Days of hospital stay | 526.32 | d |
| Travel expenses (patient, cost per km) | 0.19 | e |
| Loss of labor productivity (mean daily wage) | ||
| Under 25 years (women/men) | 25.54/30.47 | f |
| From 25 to 34 years (women/men) | 40.41/50.05 | f |
| From 35 to 44 years (women/men) | 51.24/66.80 | f |
| From 55 to 54 years (women/men) | 46.73/61.29 | f |
| 55 years and older (women/men) | 51.61/67.33 | f |
Puente Maestú L, García de Pedro J. Las pruebas funcionales respiratorias en las decisiones clínicas. Arch Bronconeumol. Mayo de 2012;48(5):161–9.
Decreto 221/2012 (31 de octubre), por el que se establecen las tarifas de los servicios sanitarios prestados en los centros dependientes del Servicio Gallego de Salud y en las fundaciones públicas sanitarias. Available from: http://www.xunta.es/dog/Publicados/2012/20121121/AnuncioC3K1-091112-0002_es.html.
Orden EHA/3770/2005 (1 de diciembre), por la que se revisa el importe de la indemnización por uso de vehículo particular establecida en el Real Decreto 462/2002 (24 de mayo), sobre indemnizaciones por razón del servicio. Available on: http://www.boe.es/boe/dias/2005/12/03/pdfs/A39852-39852.pdf.
Encuesta Anual de Estructura Salarial. Serie 2008-2012. Resultados para Galicia: Ganancia media anual por trabajador según sexo y edad. Available from: http://www.ine.es/jaxi/tabla.do?path=/t22/p133/cno11/serie/l0/&file=03004.px&type=pcaxis&L=0.
Effectiveness was measured in terms of both number of patients successfully controlled and QALYs gained 1 year after treatment in the AC. Disease control was defined as a minimally symptomatic or asymptomatic (asthma control test [ACT]≥20) patient with normal or near-normal lung function (FEV1>70% of predicted) and no exacerbations. None of the patients included in the study had completed quality of life questionnaires that would have allowed us to directly measure QALY. In order to estimate overall pre- and post-AC QALY for the series as a whole, we extrapolated QALY estimates reported in previous studies,6,17,18 using the method described in Table 1.
Statistical and Cost-Effectiveness AnalysisStatistical AnalysisThe number of controlled/uncontrolled patients at the time of their first visit to the AC and after 1 year of follow-up by a specialist were compared. Each clinical evolution parameter was also analyzed separately (ACT test score and lung function [FEV1] in liters and as a percentage of predicted at the first visit to the AC and after 1 year of follow-up, and the total number of exacerbations during the year preceding and following the first visit to the AC). In most cases, the t test for matched pairs was used to analyze the data from the same individuals at 2 different time points: point 1 (1 year preceding treatment at the AC) and point 2 (1 year following treatment at the AC). This analysis showed whether asthma management at the AC resulted in statistically significant differences in patient variables at point 1 (past status) and point 2 (current status). Non-parametric methods (for example, the Wilcoxon test) were used for non-normal distribution of certain variables. Statistical significance was set at P<.05. Data were analyzed using SPSS (IBM SPSS Statistics for Windows, version 19.0).
Cost-Effectiveness AnalysisThe incremental cost-effectiveness ratio (ICER) represents the annual incremental cost of obtaining 1 additional unit of the measure of effect if the patient is treated in the AC vs standard outpatient services. ICER is calculated using the formula: ICER=(C2−C1)/(E2−E1), where Ci and Ei are the total cost and total effect at point i (i=1: year preceding referral to the AC; i=2: year following referral to the AC). We calculated the ICER for each patient achieving disease control and the ICER per QALY gained from 2 different viewpoints: the SERGAS and the social perspective. In terms of the SERGAS, QALY considers only costs covered by the public health system (direct healthcare costs), while the social perspective includes costs met by both the patient and society (transport costs and loss of labor productivity, respectively).
ResultsPatient Demographics and Clinical and Functional StatusA total of 83 patients were included in the study (mean age 48.9±15.2 years, 60.2% women). Asthma was ruled out in 9 patients (10.8%) on the basis of a methacholine challenge test performed after suspending all the patient's usual medication. The clinical and demographic characteristics of the patients are shown in Table 3. Considering that 80% of study patients were receiving combined inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA), and that this was unsuccessful in controlling the disease in most cases, it is reasonable to assume that most study patients presented moderate to severe asthma.
Patient Demographics and Clinical Characteristics (n=83).
| Age (mean±SD) | 48.9±15.2 |
| Sex: womena | 50 (60.2) |
| Confirmation of asthma diagnosisa | 74 (89.2) |
| Onset of asthma: early (<12 years)/latea | 23 (27.7)/60 (72.3) |
| Positive prick testa | 33 (41.8) |
| IgE: UI/ml (mean±SD) | 326±907 |
| Eosinophils: number/mm3 (mean±SD) | 463±402 |
| Rhinitisa | 55 (66.3) |
| Sinusitisa | 21 (25.3) |
| Polyposisa | 11 (13.3) |
| ASA intolerancea | 7 (8.4) |
| ABPAa | 2 (2.4) |
| GERDa | 17(20.5) |
| Obesitya | 33 (39.8) |
| SAHSa | 4 (4.8) |
| Vocal cord dysfunctiona | 1 (1.2) |
| Smokinga | 10 (12.0) |
| Anxietya | 17 (20.5) |
| Depressiona | 12 (14.5) |
| Positive bronchial challengea | 35 (44.3) |
| Positive methacholine challenge: yes/no/not performeda | 8 (9.6)/12 (14.5)/63 (75.9) |
| FENO>30ppda | 52 (62.7) |
ABPA, allergic bronchopulmonary aspergillosis; ASA, acetylsalicylic acid; GERD, gastrointestinal reflux disease; IgE, immunoglobulin E; SAHS, sleep apnea hypopnea syndrome; SD, standard deviation.
Table 4 shows the evolution of the clinical and functional status of study patients. The percentage of uncontrolled patients fell significantly (59% vs 14.5%), in parallel with an improvement in clinical parameters. The total number of visits to AC physicians (including both scheduled and spontaneous consultations), visits to the emergency room, loss of working days, and days of hospitalization were reduced (the latter, however, was not clinically significant). The few cases requiring hospitalization were admitted to the pulmonary medicine department. No patients were admitted to critical care.
Clinical and Functional Evolution (Mean±SD).
| Variables | Preceding Year | Following Year | P |
|---|---|---|---|
| FEV1 (l) | 2.5±0.9 | 2.6±0.8 | <.05 |
| FEV1 (%) | 81.4±17.5 | 84.4%±16.6 | <.05 |
| ACT | 18.7±4.6 | 22.6±2.3 | <.001 |
| Exacerbations/patient | 0.8±1.6 | 0.2±0.9 | <.001 |
| Control: yes/no (%) | 41.0/59.0 | 85.5/14.5 | <.001 |
| Visits to doctor | 1.9±2.7 | 0.2±1.1 | <.001 |
| Visits to emergency room | 0.4±1.0 | 0.1±0.8 | <.05 |
| Days of hospitalization | 0.11±0.6 | 0.02±0.2 | .102 |
| Working days lost | 2.9±6.3 | 1.3±1.3 | <.05 |
ACT, asthma control test; FEV1, forced expiratory volume in 1s; SD, standard deviation.
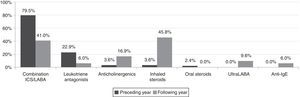
The number of patients receiving combined ICS/LABA (from 79.5% to 41.0%) and antileukotriene (from 22.9% to 6%) therapy fell, while the number receiving monotherapy ICS, omalizumab, anticholinergics and ultraLABAs (24h) increased. Systemic steroid therapy was suspended in all patients (Fig. 1).
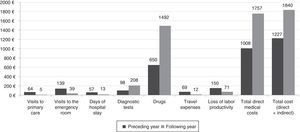
CostsManagement in the AC was associated with a significant increase in the cost of medication and diagnostic tests. This was partially offset by a reduction in the number of spontaneous consultations, hospitalizations, patient-borne travel expenses, and indirect costs related to loss of productivity. Overall, management in the AC increased the total cost per patient by 51.3% (Table 5 and Fig. 2).
Changes in Annual Costs per patient (€).
| Preceding Year (Mean±SD) | Following Year (Mean±SD) | Variation (%) | P | |
|---|---|---|---|---|
| A. Direct medical costs | 1008±959 | 1757±4662 | +74.2% | .634 |
| a) Visits to doctor, hospitalizations and emergencies | 260±669 | 57±432 | −78.1% | <.001 |
| b) Diagnostic tests | 98±94 | 208±72 | +112.8% | <.001 |
| c) Drugs | ||||
| Combination ICS/LABA | 46.24±36.29 | 22.19±31.15 | −52% | <.001 |
| Anticholinergics | 1.77±9.20 | 8.19±18.30 | +462.6% | <.05 |
| Inhaled steroids | 0.72±4.09 | 10.86±17.36 | +1501.7% | <.001 |
| UltraLABA | 0.00±0.00 | 4.82±14.85 | − | <.05 |
| Leukotriene antagonists | 4.81±8.88 | 1.27±5.03 | −73.7% | <.001 |
| Oral steroids | 0.65±5.03 | 0.00±0.00 | −100% | .180 |
| Anti-IgE (omalizumab) | 0.00±0.00 | 77±372.11 | − | <.05 |
| Total drugs | 650±518 | 1492±4630 | +129.4% | .592 |
| B. Direct non-medical costs: travel expenses | 69±191 | 12±81 | −82.6% | <.001 |
| C. Indirect costs: loss of labor productivity | 149.5±289.3 | 71.3±76.1 | −52.3% | <.05 |
| Total cost per patient (A+B+C) | 1216.5 | 1840.3 | +51.3% | .296 |
Anti-IgE, anti-immunoglobulin E; ICS/LABA, inhaled corticosteroids with long-acting beta-agonists; UltraLABA, beta-agonists lasting 24h.
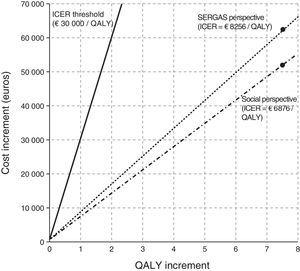
After receiving treatment at the AC, 37 previously uncontrolled asthmatics achieved disease control. Total overall QALYs gained for the series as a whole were 7.53 (mean of 0.09 per patient). From the perspective of the SERGAS, the ICER, which represents the incremental cost of obtaining 1 additional unit of the measure of effect, was €1680 per controlled patient per year, and €8265 per QALY gained. Social costs were €1399 and €6876, respectively. The foregoing shows the cost (SERGAS and social) of achieving disease control in 1 patient and 1 additional QALY, respectively (Table 6).
Cost-Effectiveness Analysis.
| Preceding Year | Following Year | Increment | |
|---|---|---|---|
| Total direct medical costs (€) | 83664 | 145831 | 62167 |
| Total social costs (direct medical cost, travel and loss of productivity) (€) | 100970 | 152745 | 51775 |
| Controlled patients | 34 | 71 | 37 |
| QALY in the controlled patient group | 30.25 (n=34) | 63.18 (n=71) | 32.93 |
| QALY in the uncontrolled patient group | 33.65 (n=49) | 8.25 8 (n=12) | −25.4 |
| Total QALY (n=83) | 63.89 | 71.42 | 7.53 (P<.001) |
| ICER from SERGAS perspectivea | |||
| ICER per controlled patient | 1680 | ||
| ICER per QALY gained | 8256 | ||
| ICER in terms social costsb | |||
| ICER per controlled patient | 1399 | ||
| ICER per QALY gained | 6876 | ||
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years, total for each patient group; SERGAS, Galician health service.
This study has shown that ACs can significantly improve the clinical status of asthma patients; in our series, the percentage of controlled patients increased from 41% to 86%, and ACT scores improved from a mean of 18.7 to 22.6. The latter is an important finding, since ACT scores, which measure the extent of asthma control, have been associated with quality of life in asthmatics.19,20 The number of exacerbations, meanwhile, fell by 75%.
All cost variables, with the exception of drugs and diagnostic tests, were significantly reduced, giving an annual saving per patient of €338. The ICER per controlled patient was €1680 for SERGAS, and €1399 for social costs. Each QALY gained by treatment received at an AC instead of in standard outpatient services represents an additional cost per year of €8256 for SERGAS, and €6876 for society. Although in Spain no official cost-effectiveness threshold has been set to determine whether or not a particular healthcare strategy should be introduced, evidence in the literature suggests that a cost of between €30000 and €45000/QALY is reasonable.21 This is in line with the recommendations of the National Institute for Health and Care Excellence (NICE), which establishes an ICER threshold of between £20000 and £30000.22Fig. 3 shows the cost effectiveness of AC treatment vs standard care.
Few studies have so far evaluated the cost effectiveness of ACs in Spain. Among these, Domingo Ribas23 in 2001 reported significant savings due to a reduction in the number of visits to the emergency room and hospitalizations. The sample size in this study, however, was small (20 patients), and all were steroid dependent.
It is important to highlight that asthma was ruled out in 10% of our patient cohort following diagnostic tests performed in the AC. This prevalence of over-diagnosis is lower than the 30% previously reported in the literature,24,25 possibly because many asthmatics had already been seen by a specialist prior to referral to the AC. It is also interesting to observe a significant reduction in combination ICS/LABA therapy in our cohort, following the recommendation of asthma guidelines, while the use of other drugs increased, like anticholinergics, inhaled steroids in monotherapy, and omalizumab, which is also in line with guidelines. If we had followed the example of other studies25 and estimated the cost of drugs over the patient's lifetime (adjusted to the life expectancy), savings in the cost of therapy would have been higher.
The higher cost of caring for asthmatics in the AC is mainly due to the cost of drugs, particularly omalizumab. This drug was used by 6% of AC patients, and increased costs by 60%. However, following asthma guidelines, omalizumab is only used in patients with severe asthma that fail to respond to inhaled treatment. The cost effectiveness of this therapy has been shown in previous studies conducted in countries with different healthcare systems.17,26–29 In Spain, a study in omalizumab reported an incremental cost of €462 per prevented exacerbation, and €26864 per QALY gained in patients receiving this therapy.30
This study has some obvious limitations. Although it is retrospective, it was conducted after full implementation of the electronic medical record system in Galicia. This ensures that information referring to changes in medication, visits to primary care, the emergency room and specialists, and sick leave are fully updated. In addition, all our patients were seen by a single specialist, thus reducing variations in clinical practice to a minimum. It is important to note that this study was conducted exclusively in a hospital setting, because treatment indications for patients not strictly catered for in clinical practice guidelines could be interpreted differently in different ACs (for example, reducing a patient's treatment or deciding which patients should receive a monoclonal antibody or a LAMA). This is a factor that could affect both the cost and effectiveness of the therapy. Although our sample size was relatively small, each patient was carefully followed-up from the AC. Furthermore, although the crossover design guarantees good pairing (each patient is self-paired), it is limited insofar as it is impossible to completely avoid possible confounding factors in the control period (prior to referral to the AC). This is because aspects such as therapeutic compliance, possible visits to a private specialist, etc., cannot be taken into account. In any event, we believe that the potential impact of seasonal confounding factors (exposure to allergens, viruses, etc.) was avoided by selecting a 12-month follow-up period, and that our detailed examination of medical records guarantees that both control and case periods were comparable in all cases.
The estimation of indirect costs is open to inaccuracies because loss of asthma-related labor productivity is calculated on the basis of the duration of an exacerbation and days of hospital stay, but does not take into account days worked despite asthma symptoms that could undermine performance in the workplace. Likewise, we had no reliable data relating to the real number of days lost to sick leave in each patient. Ojeda and Sanz de Burgoa31 estimated asthma-related loss of labor productivity in Spain, including days worked despite asthma symptoms, to amount to €258.81/month/patient. This is significantly higher than our findings in routine clinical practice (€149.5/year). This means that the real magnitude of potential savings in terms of loss of productivity is underestimated in our study, which would in turn considerably improve the social ICER.
In conclusion, the introduction of an AC in a healthcare catchment area would bring considerable benefits to patients (the percentage of well-controlled asthmatics would increase in parallel with a decrease in the number of exacerbations), and be extremely cost effective. Healthcare authorities should consider these findings when choosing the best measures to improve treatment and management of asthma patients. The creation of specialized asthma clinics is both reasonable and beneficial in terms of streamlining healthcare and improving patient outcomes.
FundingThis study was sponsored by Novartis.
AuthorshipAll the authors contributed equally to drafting the manuscript and reviewing the content, and all have approved the final version. LPLL, RV and MM had full access to the data and are responsible for the integrity and the accuracy of the statistical analysis.
Study concept and design: LPLL, AH, and RV.
Data extraction: LPLL, MCGN, and CM.
Data analysis and interpretation: MM, LPLL, RV, and AH.
Conflict of InterestDr Pérez de Llano has received fees for consultancy and for speaking at conferences or symposiums organized by the following laboratories: Novartis, GSK, Astra, Boehringer, Ferrer, TEVA, Mundipharma, Pfizer, Boehringer, Chiesi, and Rovi. The other authors declare they have no conflict of interest.
Please cite this article as: Pérez de Llano LA, Villoro R, Merino M, Neira MdCG, Muñiz C, Hidalgo Á. Coste-efectividad de una unidad monográfica de asma. Arch Bronconeumol. 2016;52:196–203.