Unexpandable lung is a mechanical complication by which the lung does not expand to the chest wall, impeding a normal apposition between the two pleural layers. The main mechanism involved is the restriction of the visceral pleura due to the formation of a fibrous layer along this pleural membrane. This happens because of the presence of an active pleural disease (lung entrapment), which can be resolved if proper therapeutic measures are taken, or a remote disease (trapped lung), in which an irreversible fibrous pleural layer has been formed. The clinical suspicion arises with the presence of post-thoracocentesis hydropneumothorax or a pleural effusion that cannot be drained due to the appearance of thoracic pain. The diagnosis is based on the analysis of the pleural liquid, the determination of pleural pressures as we drain the effusion and on air-contrast chest CT. As both represent the continuity of one same process, the results will depend on the time at which these procedures are done. If, when given a lung that is becoming entrapped, the necessary therapeutic measures are not taken, the final result will be a trapped lung. In this instance, most patients are asymptomatic or have mild exertional dyspnea and therefore they do not require treatment. Nevertheless, in cases of incapacitating dyspnea, it may be necessary to use pleural decortication in order to resolve the symptoms.
Un pulmón no expansible (PNE) es una complicación mecánica por la cual el pulmón no se expande hasta contactar con la pared torácica, impidiendo una normal aposición entre ambas hojas pleurales. El principal mecanismo implicado es la restricción de la pleura visceral al formarse una capa fibrosa a lo largo de toda esta hoja pleural. Ocurre por la presencia de una enfermedad pleural activa (pulmón en proceso de atrapamiento [PPA]), que se puede resolver si se toman las medidas terapéuticas oportunas, o remota (pulmón atrapado [PA]), en el que ya se ha formado la capa fibrosa pleural irreversible. La sospecha clínica viene dada por la presencia de un hidroneumotórax post-toracocentesis o un derrame pleural que no se puede drenar por la aparición de dolor torácico. Su diagnóstico se basa en el análisis del líquido pleural, en la determinación de las presiones pleurales a medida que drenamos el derrame y en la TC de tórax con contraste de aire. Dado que ambos representan la continuidad de un mismo proceso, los resultados dependerán del momento en que llevemos a cabo estos procedimientos. Si en el PPA no se toman con prontitud medidas terapéuticas apropiadas, el resultado final será un PA. En este, la mayoría de pacientes se encuentran asintomáticos o tienen una pequeña disnea con el esfuerzo por lo que no suelen requerir tratamiento pero, en casos de disnea incapacitante, puede ser necesario realizar una decorticación pleural para conseguir la resolución de los síntomas.
An unexpandable lung is a mechanical complication by which the lung is not able to expand to the chest wall, which impedes normal visceral and parietal pleural interaction. The pathological mechanisms that are involved include: atelectasis secondary to endobronchial obstruction, severe fibrosis of the lung parenchyma and restriction of the visceral pleura.1,2
Unexpandable lung due to restriction of the visceral pleura is subdivided into 2 categories: lung entrapment and trapped lung (Table 1). The former is due to an active process that affects the visceral pleura and is generally malignant or inflammatory in etiology. It may be progressive or it can be resolved, whether spontaneously or with specific treatment. The latter is the after-effect of a former inflammation of the pleural space that results in the formation of a fibrous membrane in the visceral pleura that does not allow for the lung to expand during extraction of the pleural liquid.3 In this case, treatment is not usually necessary and spontaneous improvement does not occur.4 The differences between lung entrapment and trapped lung will be reported throughout the article and are summarized in Table 2. There are no reports in the literature for unexpandable lung due to an atelectasis secondary to endobronchial obstruction that is able to cause a lobar collapse, nor due to severe fibrosis of the lung parenchyma. This review will center on unexpandable lung secondary to a restriction of the visceral pleura.
Differences Between Lung Entrapment and Trapped Lung.
| Lung Entrapment | Trapped Lung | |
| Mechanism | Persistent active inflammatory process that causes effusion with pleural restriction | Old inflammatory process. The pleural healing process involves the formation of a fibrous layer in the pleural surface that creates negative intrapleural pressure |
| Symptoms | Dyspnea depending on the size of the effusion | Dyspnea caused by pulmonary restriction and not by pleural effusion |
| Chest radiography | Contralateral displacement of the mediastinum | No contralateral displacement of the mediastinum |
| Pleural fluid pressure | Initially positive | Initially negative |
| Pressure/volume curve | Bimodal | Linear |
| Pleural space elastance | Normal or high | High (>14.5cmH2O/l) |
| Analysis of pleural liquid | Lymphocytic exudate | Transudate |
| Management | Depending on the underlying disease | Decortication if there is incapacitating dyspnea |
Thus, pleural inflammation due to hemothorax or to malignant or inflammatory disease can produce a certain degree of restriction of the visceral pleural, which we will refer to as lung entrapment. If these diseases are not treated in time, the mechanisms for repairing the pleura may fail, which would cause the formation of a fibrous layer along the surface of the visceral pleura. Negative intrapleural pressure would thus be created, impeding the complete apposition of both pleural layers, leading to trapped lung. Therefore, lung entrapment and trapped lung represent a continuity of the same process and, depending on at which moment complementary tests are carried out, different behavior patterns can be obtained. This can have important implications in the follow-up and treatment of these patients.
Diagnosis of Unexpandable LungThe diagnosis of unexpandable lung due to restriction of the visceral pleura (lung entrapment and trapped lung) is based on clinical suspicion in patients with either hydropneumothorax after thoracentesis or pleural effusion that cannot be completely drained due to the appearance of anterior chest pain.5 Heidecker et al. have demonstrated that unexpandable lung is the most frequent cause of pneumothorax (pneumothorax ex vacuo) after thoracocentesis.6 The mechanism by which this pneumothorax is produced after ultrasound-guided thoracentesis (a procedure by which the risk for air entering or direct damage to the visceral pleura by the catheter is under control) is believed to be due to the creation of local deformation forces in the visceral pleura as a consequence of the reduced pleural pressure (PP). This can cause small tears in the visceral pleura, with the consequent passage of air into the pleural space.6 This physiopathological mechanism has been supported by data from Cerfolio et al., where chest drains are withdrawn in post-lobectomies despite the presence of air leaks or pneumothorax.7 This has led some authors, when unexpandable lung is suspected, to prefer draining the effusion with a syringe system (as used in pleural manometry) instead of with a drain connected to a vacuum chamber, as this seems to increase the risk for pneumothorax.8 This is probably due to the fact that a vacuum will continue to drain even if the maximum PP is surpassed, while with the syringe the procedure stops as soon as the patient presents any type of symptomatology or a specific PP is reached.
Therefore, in order to differentiate between lung entrapment and trapped lung, we must know whether there is an active process affecting the visceral pleura or whether, contrarily, it is an old inflammatory process and stable over time. Lung entrapment may occur in the context of pleural effusion secondary to a neoplasm, rheumatoid arthritis, uremia, infection (complicated effusion/empyema) or after coronary artery graft bypass surgery, etc.2 The symptoms of these patients are related to the underlying disease (to which dyspnea can be added depending on the size of the effusion) and on chest radiography contralateral mediastinal shift can be observed.9,10
The diagnosis of trapped lung requires chronicity and stability over time. Diagnosis is usually late, either because it is a possibility that is not initially considered or rather because it is difficult to know how much time has transpired since the initial lesion.11 Some of the diseases associated with the development of trapped lung are coronary artery graft bypass surgery,12 empyema, hemothorax, tuberculosis, uremic pleuritis, rheumatoid arthritis, etc.13 The symptoms are usually mild or even nonexistent, and chest radiography does not present contralateral mediastinal shift.9
The diagnosis of unexpandable lung due to visceral pleural restriction is based on clinical suspicion as well as the analysis of the pleural liquid, serial determinations or PP by manometry, and thoracic computed tomography (CT). A detailed analysis of the most relevant characteristics that are observed with these procedures in order to establish the diagnosis of unexpandable lung is given below.
Pleural LiquidUnder normal conditions, in the pleural space there is a small continuous flow of liquid that forms a film about 10μm thick between the surfaces of the parietal and visceral pleuras.14 The liquid is filtered through the parietal pleura, flows toward the lower regions of the lungs and reabsorbs through the lymph nodes of the parietal pleura, fundamentally located in the diaphragmatic and mediastinal regions.15 The formation of pleural liquid depends on the difference between the hydrostatic and oncotic pressures of the pleural space and the capillaries of both pleural membranes. The movement of the liquid through each pleural layer will depend not only on the surface area and the filtration coefficient of each membrane but also the reflection coefficients for each solute, in accordance with Starling's law.16 The pleural liquid filtered through the parietal pleura comes from the systemic capillaries, while that of the visceral pleura comes from the pulmonary capillaries; therefore, the former generates a greater pressure gradient than the latter. Thus, under normal conditions, the visceral pleura plays a residual role in the entry of liquid into the pleural space.17
Animal studies18 have demonstrated that the net rate of pleural liquid formation is 0.01ml/kg/h, and the lymphatic reabsorption rate is 0.28ml/kg/h. Therefore, in order for pleural effusion to occur, it is necessary that there should be a significant increase in the production of pleural liquid (up to 28 times more to surpass the rate of lymphatic reabsorption), a reduction in lymphatic reabsorption, or both circumstances. In metastatic pleural effusion there is a release of cytokines, including vascular endothelial growth factor, which merit special attention.19 These cytokines cause, on the one hand, an increase in capillary permeability and, on the other hand, greater afflux of liquid to the pleural space. Both circumstances cause the accumulation of a liquid that biochemically corresponds with an exudate. In the case of trapped lung, two circumstances would be different from the former situation: on the one hand, there is a fibrous layer in the visceral pleura and, on the other hand, there is no vascular endothelium affectation of the pleural capillaries. The former impedes lung expansion, which generates very negative pressure in the pleural space. This difference of hydrostatic pressures between the systemic capillaries of the parietal pleura and the pleural space will cause an increase in the net flow of pleural liquid toward the pleural cavity, and therefore the difference in pressures equalizes. Thus, the liquid that is generated, as there is no increase in capillary permeability, is a transudate.
The analysis of the liquid is always useful to differentiate active inflammatory or malignant processes from chronic processes with little or no activity. For the analysis of the pleural exudates, the terms “concordant exudate” and “discordant exudate” are used, as defined by Agrawal et al.20 The former are those with a pleural fluid/serum protein ratio >0.5 and a concentration of pleural fluid lactate dehydrogenase (LDH) above 2/3 of the upper limit of normal blood levels. The discordant effusions are effusions classified as exudates by either protein ratio or pleural LDH, but not by both. At the same time, these discordant exudates can be classified as protein-discordant exudates if the pleural fluid/serum protein ratio is greater than 0.5 with a low pleural fluid LDH, or LDH-discordant exudates if the opposite is true. The interpretation of these discordances is that in the case of protein-discordant exudates there is an underlying physiopathological process of capillary leak (which could justify the high protein ratio levels); meanwhile, in the case of LDH-discordant exudates, the existing baseline process is more inflammatory in nature, which is why LDH could be high. In lung entrapment, the pleural liquid analysis shows a concordant exudate, although as the inflammatory process resolves itself the exudate may meet only one of the criteria, with a predominance of LDH-discordant exudate. Likewise, there is an increase in the total number of cells, with a predominance of neutrophils or lymphocytes according to the etiology of the pleural effusion.13 In the case of trapped lung, the pleural liquid usually acts biochemically like a transudate. However, in a recent article,13 5 of the 11 cases with trapped lung had protein levels in the pleural liquid in the range of exudates. Since trapped lung and lung entrapment represent the continuity of the same process, the authors justify this finding by the moment at which thoracentesis is done. In these cases of trapped lung, there would still be a certain protein leak due to an increase in capillary permeability. The findings of high pleural fluid LDH is an indicator of the presence of active pleural disease and, therefore, the diagnosis of PA should be questioned. But, as in the former case, the moment at which thoracentesis is carried out is important. Slightly high LDH levels could be accepted as valid, which could be reflecting a mild underlying inflammation that is disappearing.13 The liquid of the effusions due to trapped lung is usually paucicellular, with a predominance of mononuclear cells. The presence of polymorphonuclear cells or eosinophils is not consistent with the diagnosis of trapped lung, as they suggest an active pleural process. If the number of mononuclear cells is slightly elevated, the same considerations could be contemplated as in the finding of high pleural fluid LDH.13
Pleural ManometryMeasuring PP during thoracentesis (using pleural manometry) increases the safety of the patient by reducing the probability for the appearance of re-expansion pulmonary edema, which is characterized by the development of a unilateral lung edema by rapidly re-inflating a previously collapsed lung, either due to pleural effusion or pneumothorax. Although it is traditionally recommended not to withdraw more than 1–1.5l in a single maneuver, it has been seen that with pleural manometry a greater quantity of liquid can be extracted as long as PP does not reach very negative values.1,21 Furthermore, pleural manometry is used not only for the diagnosis of unexpandable lung, but also for predicting the success of pleurodesis in malignant pleural effusion.22
At functional residual capacity, the pressure in the pleural space is mildly negative (from −3 to −5cmH2O), which allows for balance between the lung elastic recoil force and the tendency of the chest wall to expand.15 This pressure is not uniform throughout all the pleural space: it becomes more negative as we ascend from the lung base toward the vertex due to the fact that the pressure gradient of the pleural liquid is different from the surface pressure. Several theories have been proposed to explain this difference, and currently the most widely accepted theory argues that pleural fluid pressure is always equal to the pressure of the pleural surface. This theory sustains that the vertical pressure gradient is the consequence of a constant, viscous flow that generates a continuous column of liquid through the pleural space that keeps the pleural membranes from coming into contact with one another. This circulation depends not only on severity but also on ventilatory and cardiogenic forces.23
The direct mediation of PP in the normal pleural space is a technological challenge since, due to the proximity of the 2 pleural membranes, the introduction of a catheter would cause geometric distortion and produce deforming forces that would not reflect the existing pleural pressure.24 Nevertheless, if there is pleural effusion, the catheter does not cause these deforming forces that influence pressure measurements, and in this case it would therefore reflect the existing pressure for this size effusion.
Pressure changes that result from the extraction of the fluid are caused by 3 factors: the elastic forces of the lung and the chest wall, altered by the changes in the geometry of the pleural space; the vertical displacement of the accumulated fluid caused by the geometric changes; and the reduced vertical extension of the accumulated liquid.22,23,25 It must be taken into account that the less fluid there is—as occurs in the final phase of pleural drainage—the greater prevalence there is of local deformation forces around the catheter, and therefore the pressure changes that are recorded at that time are conditioned by the presence of the catheter.9
The most important information that is obtained from pleural manometry is the determination of the elastic properties of the pleural cavity. Assuming this, the pressure changes in the pleural space obtained after the extraction of fluid reflect the changes in the recoil force and can be evaluated according to the quantity of fluid extracted by calculating the elastance of the pleural space (PEL) (change in pleural fluid pressure in cmH2O per liter of fluid extracted).9
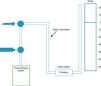
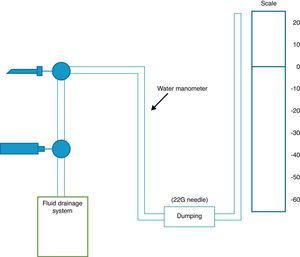
Measuring pressure in the pleural fluid can be done with a simple water column manometer, either with or without a resistor (using a 22G needle), or rather with an electronic system connected to a standard hemodynamic transducer.9 The use of a resistor with the water manometer reduces the oscillations in the column of water that are produced with respiratory movements (Fig. 1). There are currently no digital manometers on the market, so a hemodynamic transducer is used to record pleural pressure levels, as described by Doelken et al.9 In any event, there is good correlation between measurements obtained by water manometers with resistors and digital ones (r=0.97).9
Diagram of a pleural manometry system. The catheter that is introduced into the pleural space is connected to a 3-way valve that is able to measure pleural pressure or drain the effusion, according to which way the valve is turned. In the water manometer, a resistor system is installed (22G needle) to impede wide oscillation in the water column with respiratory movements, taking care that point zero of the water scale is at the same level as the catheter.
The technique has been described in detail in previous studies.9,26 It basically consists of inserting a catheter in the most apical area of the effusion to avoid the vertical force depending on the weight of the effusion and thus be able to record an initial or opening pressure that is closer to reality. Afterwards, the zero point (zero pressure) of the water column is marked at the same level where the catheter was inserted, although the distal end may be below. After measuring the initial pressure, pleural fluid is withdrawn through another tube system connected to the catheter, and mean pleural pressure is measured once again, and so on. There are different protocols regarding how much fluid to withdraw for each measurement. At the beginning of the test, pressure can be measured after draining 250cc, but once we have withdrawn an important quantity of liquid, it is more prudent to do so every 50–100cc in order to avoid an abrupt drop in PP.9,27
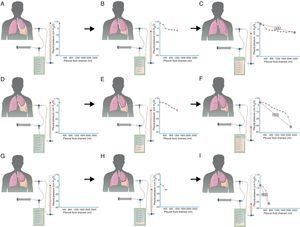
The initial or opening pressure is a measurement that can tell us whether a lung will expand normally, partially (lung entrapment) or not at all (trapped lung). In normal lungs, initial PP is slightly positive and, as the fluid is extracted, PP slowly descends, which suggests that the lung is progressively returning to its place until apposition of both pleural membranes is reached, if all the fluid can be removed (Fig. 2A–C). In the case of lung entrapment, the initial PP is also usually positive and the first part of the curve behaves like a normal lung. However, at some moment PP falls quickly because the lung, which is in entrapment phase, is not able to expand more. This is known as a bimodal curve (Fig. 2D–F). Lastly, in PA, in which there is a fibrous layer along the visceral pleura that impedes lung expansion, initial PP is negative and drops rapidly from the start as fluid is withdrawn (Fig. 2G–I).
In a pleural effusion with a normal lung (A), initial PP is mildly positive. As the liquid is withdrawn (B), PP falls slowly and the lung expands progressively. Once all the effusion is withdrawn (C), the lung comes into contact with the chest wall and PEL becomes normal. In lung entrapment, the visceral pleura has a slight thickening and the initial PP is, as in a normal lung, mildly positive (D). When the fluid is drained, the lung expands progressively and PP slowly descends (E). At this point, the lung becomes trapped and cannot expand more; PP will drop rapidly, producing elevated PEL with a bimodal pressure/volume curve (F). In trapped lung, the visceral pleura has a thicker layer of fibrin that keeps the lung from expanding, and therefore initial PP will be negative (G). The withdrawal of the liquid, on the one hand, and the lung rigidity, on the other hand, cause PP to descend rapidly (H), which leads to high PEL (I).
The elasticity of the pleural space, meaning the ability of the lung to return to its natural position as pleural fluid is withdrawn, can be measured with PEL. To do so, a diagram with pressure/volume curves is constructed. In the case of an expandable lung, a monophasic curve is obtained in which the pressure slowly drops as the fluid is extracted and the PEL is ≤14.5cmH2O/l9,27,28 (Fig. 2C). In lung entrapment, the curve is biphasic or bimodal: based on a slightly positive initial PP, PP falls slowly and during this phase PEL is normal. Later, PP starts to drop rapidly because the lung cannot expand more and PEL rises (Fig. 2F). Last of all, in trapped lung the curve is also monophasic. Initial PP is negative and falls quickly from the beginning, and therefore PEL is high (Fig. 2I). PEL >14.5cmH2O/l suggests unexpandable lung and values greater than 19cmH2O/l are related with failed pleurodesis.22
Pleural manometry is a valuable tool for documenting the abnormal mechanics of the pleural space but, alone, it is not diagnostic for trapped lung because a high PEL can also be observed in lung entrapment due to a malignant or inflammatory disease. Likewise, this technique allows us to perform evacuation thoracentesis without the risk of producing edema due to lung re-expansion since, at that time, PP would descend rapidly and the procedure should be suspended. Three studies that used pleural manometry to control therapeutic thoracentesis and included more than 350 patients did not describe any cases of edema due to lung re-expansion.13,26,27 Two studies done in animals demonstrated that the development of edema due to lung re-expansion correlates with the duration of the effusion and with excessively negative pleural pressures.29,30 Based on these results, it is assumed that a pressure that does not exceed −20cmH2O is reasonably safe, while a pressure of −40cmH2O is high risk for lung edema. In malignant pleural effusion, pleural manometry helps predict the success of pleurodesis, as PEL values higher than 19.5cmH2O are associated with failure and it would be more convenient to use other therapeutic methods, like the placement of a permanent catheter. Lastly, if during therapeutic thoracentesis there is an abrupt drop in PP and the patient develops chest pain or dyspnea, pleural manometry can reestablish PP in the physiological range by being able to induce pneumothorax, letting air enter into the pleural space in a controlled manner through the system itself until this is achieved.13
The prevalence of unexpandable lung is not insignificant. In a series of 291 patients who underwent therapeutic thoracentesis controlled with pleural manometry, one out of every 3 (93/291; 32%) presented unexpandable lung due to pleural restriction. A total of 52 cases had trapped lung, and 41 had lung entrapment. Of these latter, 66% were related, directly or indirectly, with malignant disease. Therefore, it seems reasonable when the authors suggest that, in patients with therapeutic thoracentesis, it is convenient to routinely measure PP due to its prognostic and therapeutic implications.31
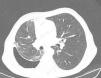
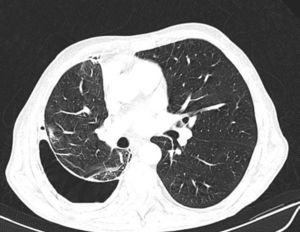
Chest Computed TomographyAs previously mentioned, patients with lung entrapment or trapped lung present thickening of the visceral pleura. However, it is very difficult to observe on chest radiography, or even conventional CT, as the thickness is usually less than 3mm. In order to demonstrate pleural thickening, Huggins et al. suggested performing what they call CT with air contrast (Fig. 3). The technique entails creating pneumothorax during pleural manometry in those patients who meet the following criteria: appearance of PP (less than −25cmH2O) and exclusion—due to the clinical history and analysis of the pleural fluid—of malignancy or active pleural inflammation. The pneumothorax is able to determine the thickening of the visceral pleura and, in addition, allows for fluid to keep being drained, if necessary, and alleviates the chest pain generated by excessively negative pressures. The procedure consists of opening a valve in the manometer circuit in order to allow air to enter into the pleural space until physiological pleural pressures are reached (mean pleural liquid pressure −5cmH2O). This procedure can only be done with monitoring and the use of a digital manometer. Even so, the demonstration of pleural thickening, the persistence of pneumothorax in conditions of negative PP and high PEL, in the absence of other causes of unexpandable lung, establish the presence of some consistent mechanical conditions (although not diagnostic) of trapped lung.13
In brief, the diagnosis of unexpandable lung due to restriction of the visceral pleura is based on a situation of clinical suspicion (post-thoracentesis hydropneumothorax or pleural effusion that cannot be drained due to the appearance of thoracic pain) and it is necessary to analyze the pleural fluid, determine pleural pressures as the effusion is drained and order an air-contrast chest CT. Since lung entrapment and trapped lung represent the continuity of a same process if allowed to evolve, the results that are obtained will depend on the moment these procedures are done. Pleural effusion in lung entrapment will behave biochemically like an exudate and will run its course with a higher number of cells (neutrophils or lymphocytes). If it turns into trapped lung, the characteristics of the fluid will be those of a transudate with a small cell content (lymphocytes). In this transformation, effusions can be found with a protein level in the range of exudates or slightly high LDH. The initial PP in the case of lung entrapment is slightly positive and the first part of the curve behaves like that of a normal lung. But as the fluid is withdrawn, PP drops rapidly because the lung becomes trapped, the curve has a bimodal shape and the PEL is either normal or high. In trapped lung, the initial PP is negative and the curve descends rapidly as the fluid is drained with a high PEL. Last of all, air-contrast chest CT confirms that thickening of the visceral pleura and the persistence of pneumothorax can be observed in conditions of negative PP.
TreatmentIn lung entrapment, it is fundamental to treat the underlying process that is causing the pleural effusion as soon as possible. In the case of hemothorax, it must be drained quickly, and in pneumonia associated with parapneumonic effusion, early antibiotic treatment must be established and a thoracic drain inserted if the effusion is complicated. In symptomatic malignant pleural effusion, and taking into account that lung entrapment involves decreased effectiveness of pleurodesis,22 the first therapeutic option would be to insert a permanent pleural drain.32 If the appropriate therapeutic measures are not taken promptly, the final result will be trapped lung.
In trapped lung, most patients are asymptomatic or perhaps have small dyspnea with exertion, and they therefore do not usually require treatment. The persistence of pleural effusion should not lead to repeated evacuation thoracenteses, because the fluid would accumulate rapidly in the same quantity as before being withdrawn in an attempt to “normalize” the negative PP. In the cases in which dyspnea is incapacitating for the patient, it is very important to exclude other causes before carrying out pleural decortication. Some authors argue that trying to achieve lung re-expansion with thoracostomy tubes should only be attempted in symptomatic patients with trapped lung and who are poor candidates for surgery.13
Conflicts of InterestThe authors declare having no conflicts of interest.
The authors would like to thank Fernando Vázquez Vázquez for his help with the diagrams.
Please cite this article as: Pereyra MF, et al. Pulmón no expansible. Arch Bronconeumol. 2013;49:63–9.