COVID-19, a viral disease caused by the SARS-CoV-2, is a lethal infection in a significant number of cases with several clinical manifestations and symptoms, such as lung damage, exacerbated inflammatory response and, in many cases, generalized organ failure.1 To combat these symptoms, one or more immunosuppressive drugs can be used individually or in combination2; therefore, the treatment itself can impose an additional risk for the development of fungal infections. It is important to emphasize that there are other associated risk factors, such as malnutrition, prolonged intubation, central and/or arterial venous access, and the need for a nasogastric tube that can increase the chances of mycosis infections in patients suffering from severe cases of COVID-19.3 In the past few decades, the world has experienced an increase in the incidence and spread of emerging fungal infections. This scenario has caused a fundamental change in the epidemiology of invasive fungal diseases, especially in immunocompromised individuals, such as those affected by human immunodeficiency virus (HIV), cancer, or undergoing transplants.4 Among these emerging pathogens, fungal infections caused by Trichosporon asahii have been identified in neutropenic cancer patients, which significantly increased the severity of their cases leading to a high mortality rate. Recently, the infection has also been identified in other groups of immunocompromised patients.5
Here, we report a case of an immunocompetent patient suffering from a severe case of COVID-19 infection who also developed a triple pulmonary coinfection with Pseudomonas sp., Stenotrophomonas sp. and Trichosporon sp.
The patient reported in this study was a 58-year-old male patient that, in 2000, presented a mild chronic kidney disease and had a tuberculosis infection treated with isoniazid chemoprophylaxis. In 2004, he was diagnosed with a bladder neoplasm and was submitted to a radical cystoprostatectomy and a left nephrectomy due to a renal metastasis. The patient had been free of malignant disease for the past ten years and had a competent immune status.
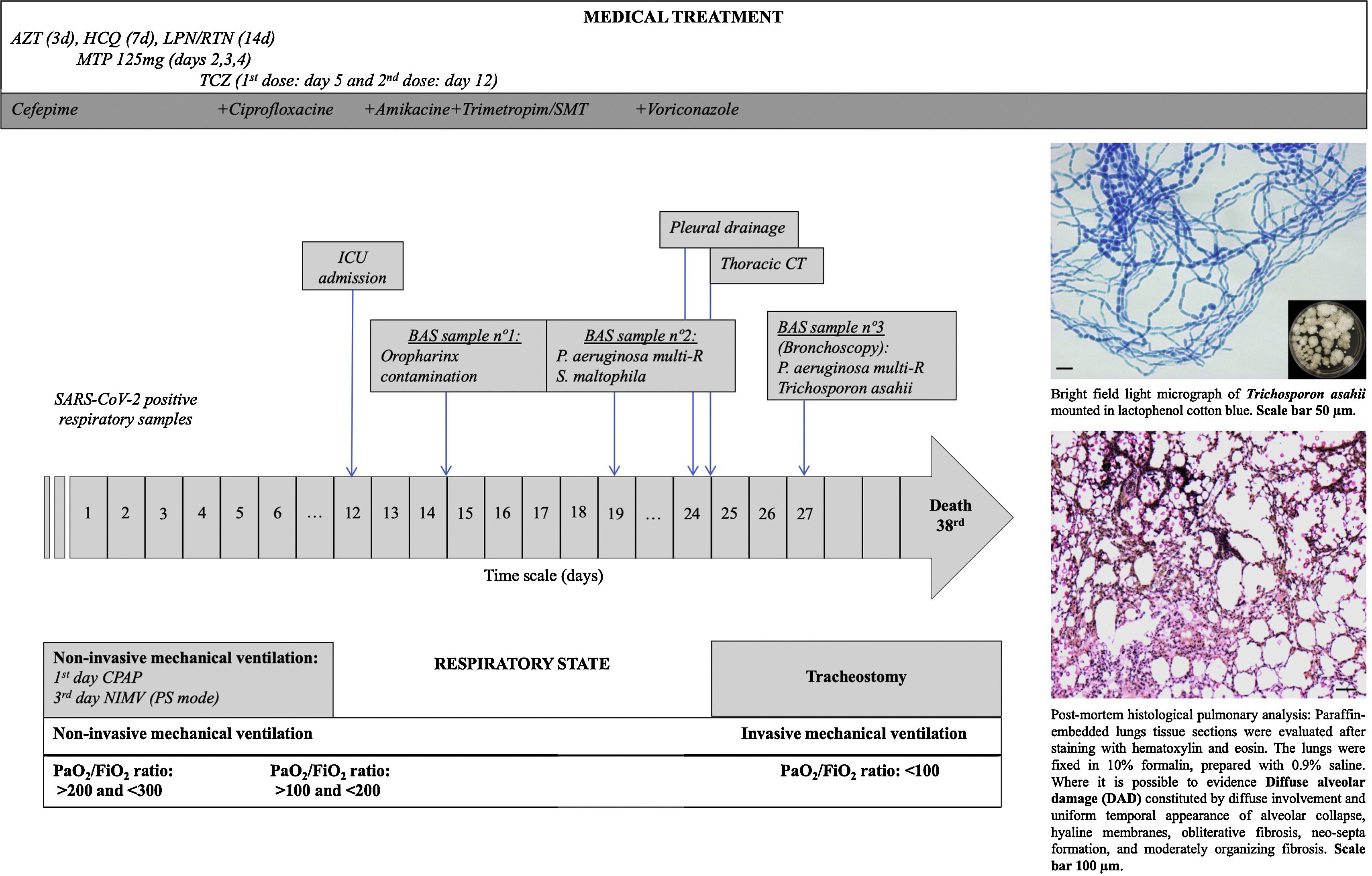
The patient went to the emergency room (Fig. 1 – Day 1) after presenting, for twelve days, high fever, unproductive cough, and general affection. Twenty-four hours before going to the hospital, the patient presented dyspnea with moderate efforts (∼90% SpO2 at rest). A blood test confirmed lymphopenia (900/mm3), high C-Reactive Protein (CRP=35mg/dL), and altered transaminases (Glutamic Oxaloacetic Transaminase (GOT) 160IU/L; Glutamate Pyruvic Transaminase (GPT) IU/L; Gamma Glutamyl Transpeptidase (GGT) 75IU/L, and Phosphatase Alkaline (PAL) 60IU/L). Chest X-ray images revealed an infiltration in the left base of the lung and the COVID-19 test was positive. With the diagnosis of pneumonia secondary to SARS-CoV-2, the patient was admitted to the Intensive Care Unit (ICU). A treatment off-label with Hydroxychloroquine sulfate 400mg (equivalent to 310mg base) Q12H for 2 doses, followed by 200mg (=155 base) Q12H for 5 days), Lopinavir/Ritonavir (400mg/100mg), Azithromycin (500mg daily for three days), Methylprednisolone (bolus of 125mg daily for three days), and Tocilizumab (two doses of 8mg/kg IV – 8h apart) was prescribed and initiated.
Timeline for an immunocompetent COVID-19 patient who developed an invasive pulmonary trichosporonosis by Trichosporon asahii. The patient was admitted to the intensive care unit on the 12th day after a positive diagnosis by SARS-CoV-2 and died after 24 days. Abbreviations: AZT: Azithromycin HCQ: Hydroxychloroquine sulfate LPN/RTN: Lopinavir/Ritonavir MTP: Methylprednisolone TCZ: Tocilizumab BAS: Bronchial Aspiration CT: Computer Tomography Multi-R: Multi-resistant ICU: Intensive Care Unit CPAP: Continuous Pressure in Upper Airway NIMV: Non-Invasive Mechanical Ventilation.
During hospitalization (Fig. 1), the respiratory function of the patient aggravated and a high oxygen flow [50 LPM and 90% Fraction of Inspired Oxygen (FiO2)] was needed. Finally, an orotracheal intubation and an invasive ventilation was needed on the 12th day of hospitalization in the ICU. On the 19th day (Fig. 1), multiresistant Pseudomonas aeruginosa and Stenotrophomonas maltophila strains were isolated from the respiratory secretions of the patient. Despite the treatment with antibiotics, the clinical course of the infection had a poor outcome, and the patient presented persistent fever and hypoxemic respiratory failure. In a chest radiographic exam, left pneumothorax was detected and a chest drainage was performed. A chest CT scan was performed and a suspected pleuro-pulmonary fistula in the lingular zone, a moderate pleural effusion, and bilateral infiltrates were visualized. Following these results, a fiber bronchoscopy was performed in which abundant purulent secretions, mainly in the right bronchial tree, were visualized and aspirated. Endobronchial lesions were not identified. In the collected respiratory secretions, Trichosporon asahii was isolated and a treatment with antifungal drugs (voriconazole) started in day 28th (Fig. 1). The clinical response was weak and the patient developed a septic shock and died on the 38th day after his transfer to the ICU (Fig. 1). The family of the patient authorized the autopsy. The pulmonary autopsy study confirmed the existence of a pattern of acute alveolar damage.
Every day, a discovery regarding the pathophysiological behavior of SARS-CoV-2 emerges. The SARS-CoV-2 causes a lower respiratory infection which in turn can lead to Acute Respiratory Discomfort Syndromes (ARDS). In addition to a diffuse alveolar damage with severe inflammatory exudation, patients with COVID-19 can present immunosuppression with decreased CD4+T and CD8+ T cells.6 This clinical scenario opens the door for the development of coinfections by opportunistic microorganisms. Within this context, different published reports have shown the importance of assistant doctors and laboratory specialists in verifying the occurrence of potential coinfections, such as aspergillosis, candidiasis, mucormycosis, or cryptococcosis that could lead to co-morbidities in patients with COVID-19.7
The incidence of invasive infections caused by opportunistic fungal species has increased in recent decades. These fungi are normally difficult to diagnose, resistant to many antifungals, and are associated with high mortality rates.8 In the 1980s, the invasive Trichosporon infection was considered the second most common cause of fungemia among immunosuppressed patients who also suffered from hematological diseases, hemochromatosis, end-stage renal disease, or who were on a long-term corticosteroid treatment. Depending on the age, underlying conditions, presence of neutropenia, and clinical treatments, the mortality rates of patients suffering from an invasive tricosporonosis infection can range from 30% to 90%.9–11 Until now, our case is the first report that shows a Trichosporon infection in a COVID-19 patient.
In conclusion, we report a case of a triple pulmonary coinfection in an immunocompetent patient with severe SARS-CoV-2 pneumonia. As the pandemic continues to spread around the world, other reports to assess the frequency of emergent and reemerging highly resistant bacterial and fungal coinfections in individuals suffering from COVID-19 are needed. These coinfections impose severe complications in COVID-19 patients that might lead to death due to the aggravation in the primary viral condition.
Ethics declarationsThe present study was approved by the Ethics Committee of the Fundación Jiménez Díaz Health Research Institute (EO102-20-HRJC). Due to the pandemic situation, informed consent was not requested from the patients. Personal information and data obtained from the subjects were kept confidential.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We acknowledge Dr. Barbara Hissa for critical reading and scientific editing of the manuscript. This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001 and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).