Bronchiectasis is currently growing in importance due to both the increase in the number of diagnoses made as well as the negative impact that its presence has on the baseline disease that generates it. The fundamental aspects in these patients are the colonization and infection of the bronchial mucous by potentially pathogenic microorganisms (PPM), which are the causes in most cases of the start of the chronic inflammatory process resulting in the destruction and dilatation of the bronchial tree that is characteristic in these patients. The treatment of the colonization and chronic bronchial infection in these patients should be based on prolonged antibiotic therapy in its different presentations. Lately, the inhaled form is becoming especially prominent due to its high efficacy and limited production of important adverse effects. However, one must not overlook the fact that the management of patients with bronchiectasis should be multidisciplinary and multidimensional. In addition to antibiotic treatment, the collaboration of different medical and surgical specialties is essential for the management of the exacerbations, nutritional aspects, respiratory physiotherapy, muscle rehabilitation, complications, inflammation and bronchial hyperreactivity and the hypersecretion that characterizes these patients.
Las bronquiectasias presentan actualmente una importancia creciente tanto por el incremento en el número de diagnósticos que se realizan como por el impacto negativo que su presencia supone sobre la enfermedad de base que las genera. Un aspecto fundamental en estos pacientes es la colonización e infección de la mucosa bronquial por microorganismos potencialmente patógenos (MPP), causante en la mayoría de los casos del inicio del proceso inflamatorio crónico que termina con la destrucción y la dilatación del árbol bronquial que caracteriza a estos pacientes. El tratamiento de la colonización y de la infección bronquial crónica en estos pacientes se debe basar en la terapia antibiótica prolongada en sus distintas presentaciones, de las cuales la forma inhalada está adquiriendo un especial protagonismo en los últimos tiempos por su elevada eficacia y su escasa producción de efectos adversos importantes. Sin embargo, no debe pasarse por alto que el manejo del paciente con bronquiectasias debe ser multidisciplinar y multidimensional, dado que además del tratamiento antibiótico es imprescindible el trabajo de diferentes especialidades médicas y quirúrgicas para el manejo de las agudizaciones, los aspectos nutricionales, la fisioterapia respiratoria, la rehabilitación muscular, las complicaciones, la inflamación e hiperreactividad bronquial y la hipersecreción que caracteriza a estos pacientes.
Bronchiectasis is the final stage of lung damage that is caused by dozens of diseases, both systemic as well as local.1–5 Although until some years ago it was thought that bronchiectasis was becoming extinct, was no more than a thing of the past and a consequence of the infectious epidemics of other eras, today it is accepted that the number of diagnoses is quickly growing. Among other reasons, this is in part due to the greater longevity (enabling a greater chronicity of the generating diseases), but it is especially thanks to the reliability of the high-definition topography techniques that are currently and routinely used.6–8 However, the true dimension of bronchiectasis is found not only in the deterioration that it generates in patient quality of life but also in the negative prognostic impact that it adds to the disease that generates it.9–11 From a pathogenic standpoint, the most frequent mechanisms in the formation of bronchiectasis have been known since the 1980s.12,13 An initial aggression in the bronchial mucosa, usually due to an infection produced by a potentially pathogenic microorganism (PPM), unleashes a chain of events that end up with the progressive destruction of the bronchial wall and the characteristic dilation of the airway lumen that defines this disease. The intermediate mechanisms that wind up causing this destruction are fundamentally derived from previous damage to the defense mechanisms, either genetically (as occurs in many diseases) or by acquired destruction. This can be a consequence of the lytic products segregated by the neutrophilic and mononuclear inflammation caused by the infection as well as the secretion of toxic substances by the PPM themselves that perpetuate a situation of chronic infection and inflammation that wind up closing the vicious circle, ensuring the progression of the disease. The final consequence is the progressive airway obstruction and the appearance of the typical symptoms of this disease, especially chronic hypersecretion and the more advanced stages of dyspnea, all of which modulate the progressive loss of lung function and quality of life of patients, leading to early death.14
Throughout the natural history of bronchiectasis, there is a fact that defines an important turning point from the evolutionary standpoint: the appearance of colonization in the bronchial mucosa by PPM, especially when this situation becomes chronic and generates an increase in the symptoms of the patient. But without a doubt, from among the possible PPM that may colonize the mucosa of patients with bronchiectasis, there is one that stands out from the rest due to its extreme virulence: Pseudomonas aeruginosa (PA). What is still up for debate is the relationship between the presence of bronchial colonization, especially by PA, and the later deterioration of the disease. Some authors postulate that its presence means a later progressive deterioration of the disease in terms of causality,15 meanwhile others suggest that this microorganism is only a marker for severity that appears in the more severe forms of the disease due to the previous destructuring of the bronchial mucosa.16 Whichever may be true, it seems that there is an agreement that the isolation of PA in the bronchial mucosa of patients with bronchiectasis does not foretell a good evolution, given that they are related with a greater number and severity of exacerbations, poorer quality of life, greater volume and purulence of sputum, greater deterioration of lung function and, in short, poorer vital prognosis.17–20 Along these lines, the two guidelines for bronchiectasis treatment that are currently in effect—one promoted by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)21 and the other by the British Thoracic Society (BTS),22 both recently published—coincide in indicating that the appearance of PA in the bronchial mucosa of these patients, within the different types of colonization, should be treated aggressively and early, especially by means of a more or less prolonged antibiotic therapy. However, it should not be forgotten that the treatment of bronchiectasis and bronchial colonization must be multidisciplinary. Moreover, even though antibiotic treatment is the cornerstone of the treatment, it does not always achieve the optimal control of the patient and requires the support of these other adjuvant treatments, whose main function is to improve the general symptoms of the patient either by reducing bronchial inflammation (anti-inflammatories), improving symptoms (bronchodilators), aiding expectoration (physiotherapy and mucolytics) or improving the general state (physical exercise, rehabilitation and nutrition). The present review is a practical overview of the global management of patients with non-cystic fibrosis (CF) bronchiectasis, while it also discusses the different varieties of colonization and bronchial infection that affect these patients, within the framework of the current guidelines.
Treatment of Bronchial Colonization in Patients With BronchiectasisConcepts of Colonization and Bronchial Infection in BronchiectasisAs a consequence of the already mentioned structural alterations, bronchiectasis generates a micro-environment that is ideal for the growth of PPM whose existence is perpetuated by their capacity for developing defense mechanisms and hindering the action of the immune system and antimicrobials (hypermutability, formation of capsules or biofilm, etc.). The quantity of bacteria at a given time, the situation of the immune defense system, the invasive capacity of the PPM and the action of the antibacterials will determine different situations whose characterization is important given that they present therapeutic implications.23,24 Bronchial colonization is defined as the presence of a bacterial population in the bronchial mucosa that does not induce an inflammatory response with clinical repercussions, except for an increase in the expectoration of mucus. Depending on the identification and permanence of the PPM in the respiratory samples, the colonization may be: initial, in the case of a first positive culture, outside a process of exacerbation, and not isolated in previous periodical cultures; intermittent, in the case of alternating positive and negative cultures for a same PPM, with at least one month between them (usually reflecting a low-grade chronic bronchial colonization or a small number of colonies that are occasionally not detected in sputum); and chronic, when the same PPM is detected in 3 or more consecutive cultures separated by at least one month during a period of 6 months without concomitant antibiotic treatment.
Chronic bronchial infection entails a situation in which a bronchial colonization generates an inflammatory response that provokes the appearance of clearly discernible symptoms in the patient, generally chronic purulent expectoration. It is usually accompanied by a systemic affectation and an increase in the number of exacerbations.25,26
Treatment of Initial Bronchial ColonizationBefore commenting on the treatment of initial bronchial colonization, it is important to mention that there is currently no indication for prophylactic antibiotic treatment administered periodically in patients with non-CF bronchiectasis and high risk for colonization by PPM, including PA, although studies are needed to determine the cost-effectiveness of this type of treatment.22
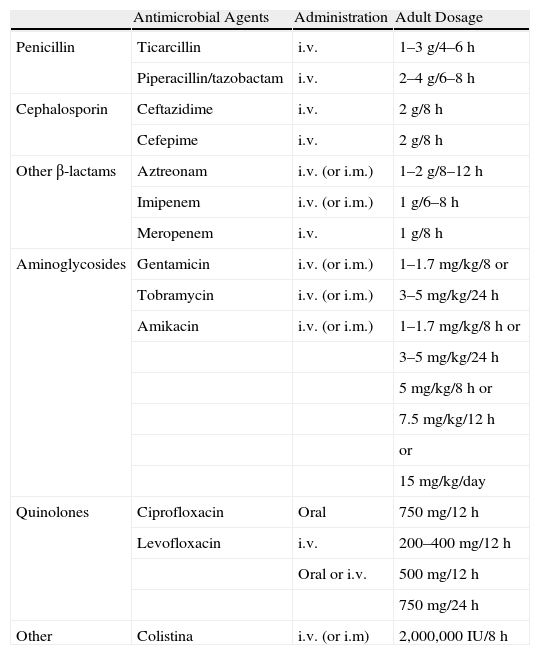
In spite of the fact that there is limited scientific evidence in patients with non-CF bronchiectasis (there is more for patients with CF), it is accepted that, given the negative effects of PA on different clinical, functional and evaluative parameters in patients with any type of bronquiectasis,17–20 the growth of this PPM in the first culture of a respiratory sample should suggest the use of intense antibiotic treatment. At least in theory, this would suppose the last chance to eradicate PA from the bronchial mucosa, which becomes even more improbable once this microorganism chronically colonizes the airways. The most recommended treatment is based on the use of 750mg every 12h of oral ciprofloxacin for 3 weeks. The addition of an inhaled antibiotic (additive-free tobramycin or sodium colistimethate) over a longer term (3–12 months) should be considered in the case of lack of efficacy of the oral treatment determined by the persistence of PA in the cultures of the respiratory samples in later control testing. An alternative to ciprofloxacin is the use of two intravenous antibiotics with antipseudomonal activity for 14–21 days (Table 1). For the remaining PPM, there is no scientific evidence that supports antibiotic treatment in this situation, and treatment should therefore be individualized.21 The BTS also recommends antibiotic treatment depending on the antibiogram in the initial colonization by Staphylococcus aureus resistant to methicillin due to the negative impact on the patient.27
Systemic Antibiotics With Activity Against Pseudomonas aeruginosa Used in Patients With Bronchiectasis and Recommended Dosage.
| Antimicrobial Agents | Administration | Adult Dosage | |
| Penicillin | Ticarcillin | i.v. | 1–3g/4–6h |
| Piperacillin/tazobactam | i.v. | 2–4g/6–8h | |
| Cephalosporin | Ceftazidime | i.v. | 2g/8h |
| Cefepime | i.v. | 2g/8h | |
| Other β-lactams | Aztreonam | i.v. (or i.m.) | 1–2g/8–12h |
| Imipenem | i.v. (or i.m.) | 1g/6–8h | |
| Meropenem | i.v. | 1g/8h | |
| Aminoglycosides | Gentamicin | i.v. (or i.m.) | 1–1.7mg/kg/8 or |
| Tobramycin | i.v. (or i.m.) | 3–5mg/kg/24h | |
| Amikacin | i.v. (or i.m.) | 1–1.7mg/kg/8h or | |
| 3–5mg/kg/24h | |||
| 5mg/kg/8h or | |||
| 7.5mg/kg/12h | |||
| or | |||
| 15mg/kg/day | |||
| Quinolones | Ciprofloxacin | Oral | 750mg/12h |
| Levofloxacin | i.v. | 200–400mg/12h | |
| Oral or i.v. | 500mg/12h | ||
| 750mg/24h | |||
| Other | Colistina | i.v. (or i.m) | 2,000,000IU/8h |
i.v.: intravenous; i.m.: intramuscular; (): scarcely used administration method.
The treatment should be based on the prolonged administration of antibiotics given the appearance of one of the following situations: intermittent or chronic colonization by PA, repeated exacerbations (according to the BTS guidelines, at least 3 exacerbations per year with the need for systemic antibiotic treatment), early relapses, hospitalizations or accelerated deterioration in lung function (in these last four cases, regardless of the PPM that causes the situation). The guidelines to be followed are the same for chronic bronchial infection,21,22 as seen below.
Treatment of Chronic Bronchial InfectionIn this case, the treatment is aimed at breaking the vicious pathogenic circle of infection–inflammation of the airway, reducing the bacterial load and the inflammatory response, thus reducing the volume and purulence of the sputum as well as the number and severity of the exacerbations. Another aim is attempting to stop the loss of pulmonary function, as only on rare occasions (especially in the case of PA) will long-term eradication of the microorganism be achieved. The treatment is based on the administration of long-term antibiotic treatment, in the same way as in intermittent colonization, given the presence of a chronic infection by PA in all the cases or by another other PPM if there are repeated exacerbations, early relapses, hospitalizations or an accelerated deterioration in the lung function. As for the dosage, several studies have analyzed the effectiveness of different prolonged antibiotic treatments with disparate results depending on the type of administration: antipseudomonal oral treatment (usually fluoroquinolones like ciprofloxacin or levofloxacin); intravenous treatment (ceftazidime, piperacillin–tazobactam, imipenem, aminoglycosides or aztreonam) or rather prolonged inhaled antibiotic treatment (tobramycin or colistin). A fourth option in more severe patients is the combination of two of the three former options, usually inhaled antibiotics plus systemic antibiotics (oral or intravenous). Although there is no clear scientific evidence about what the choice should be, as has been mentioned, inhaled antibiotics offer certain advantages that make many professionals choose them as a first treatment option (see Inhaled antibiotics). The final choice of the type of antibiotic logically should depend on the PPM isolated and its antibiogram. The treatment should be maintained until the control of the infection is reached based on sputum that is as mucous as possible or on a reduction in exacerbations.21,22
Systemic AntibioticsThe first studies that were done dealt with the effect of long-term antibiotics in the treatment of chronic colonization-infection in patients with bronchiectasis with the use of amoxicillin, tetracycline, gentamicin, amoxicillin or ciprofloxacin.28–38 A systematic review by Evans et al. in 200328 concluded that long-term therapy with systemic antibiotics for the treatment of chronic colonization achieved a general improvement in symptoms but had no effect on the lung function or either the number or severity of the exacerbations of the patients, while it did not clarify its effect on mortality. Nevertheless, an increase has been observed in the resistances of some systemic antibiotics administered over the long-term for PA, especially fluoroquinolones; therefore, according to the guidelines of the BTS, the repeated use of cycles of this family of antibiotics should be avoided under these conditions.
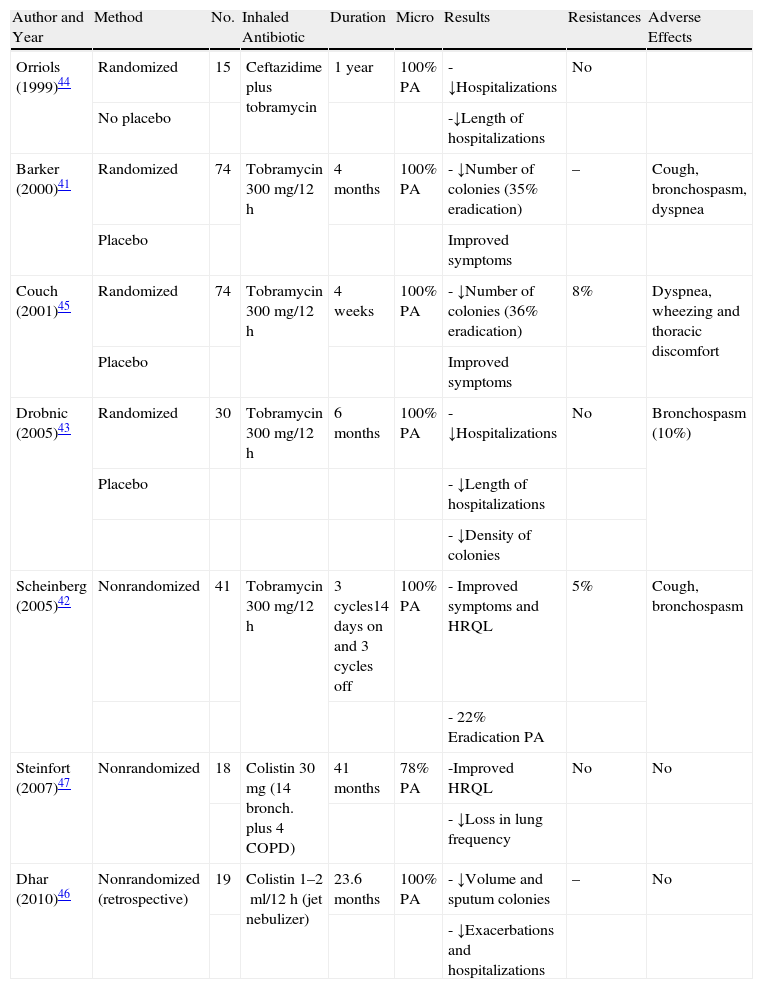
Inhaled AntibioticsSome authors have demonstrated that the concentrations of antibiotics reached in the respiratory secretions is up to 20 times higher in inhaled therapy than in systemic therapy, which could imply greater efficacy, a lower rate of systemic side effects and, therefore, the possibility for safely prolonging the treatment time.39,40 In patients with non-CF bronchiectasis, until now the formal indication of inhaled antibiotic therapy has not been approved and should be requested as compassionate medication. However, there are different studies that coincide in pointing out that the treatment with inhaled antibiotics is effective in reducing the density of PA colonies in sputum and in the improvement of certain clinical aspects41–47 (Table 2). The rates for prolonged eradication of PA with inhaled antibiotic treatment, and also with systemic treatment, are quite variable. The majority of authors coincide in indicating that, after withdrawing the medication, the rate of recurrence is nearly universal. Some recent reviews and the current guidelines recommend the use of inhaled antibiotics in patients with non-CF bronchiectasis and chronic bronchial infection/colonization by PA (for the chronic infection by other microorganisms, the indication should be individualized) or given the presence of adverse effects, resistances or inefficacy of the oral long-term treatment, as long as extreme care is taken in monitoring side effects and the effectiveness of the treatment,21,22,48,49 even being able to combine both methods of administration (inhaled and systemic) in some cases. Both in CF as well as in the rest of etiologies, the appearance of resistances of PA to tobramycin has been reported with the inhaled use of this drug, which may disappear after the temporary suppression of the treatment. Some authors have referred less frequency (only 5%) of PA resistances with the use of sodium colistimethate.47 The pharmacological characteristics of intravenous preparations are not ideal for inhalation, especially with regards to its osmolarity, pH and presence of substances that are airway irritants. The tobramycin solution for nebulizer (TOBI®, Novartis; Bramitob®, Chiesi) and sodium colistimethate (GES®, G.E.S. Genéricos Españoles Laboratorio; Promixin®, Praxis Pharmaceutical) are the two antibiotics available on the market apt for inhaled use in patients with bronchiectasis (formal indication in CF and compassionate indication in bronchiectasis of other origins). It is administered by means of jet nebulizers (Pari LC Plus®) or dynamic or static mesh electronic nebulizers (eFlow rapid® and I-neb®).50 In comparison with the jet nebulizers, the mesh ones are less voluminous, more silent, faster and more portable. The treatment with the tobramycin solution for inhalation should be done at a dosage of 300mg/12h at alternating 28-day cycles. The sodium colistimethate is usually used at a dosage of 2 million IU/12h dissolved in 4ml of a solution that is as isotonic as possible, although with the use of the I-neb nebulizer by Respironics® the dose can be reduced to 1 million IU/12h as the medication is released only during the inspiration of the patient and not continuously as in the rest of nebulizers. Unlike inhaled tobramycin, this drug is usually used without rest periods. Treatment with inhaled antibiotics for chronic PA colonization/infection should be maintained as long as an acceptable risk/benefit ratio is achieved.
The Most Important Studies on the Use of Inhaled Antibiotics for the Treatment of Chronic Bronchial Colonization-Infection in Patients With Bronchiectasis.
| Author and Year | Method | No. | Inhaled Antibiotic | Duration | Micro | Results | Resistances | Adverse Effects |
| Orriols (1999)44 | Randomized | 15 | Ceftazidime plus tobramycin | 1 year | 100% PA | - ↓Hospitalizations | No | |
| No placebo | -↓Length of hospitalizations | |||||||
| Barker (2000)41 | Randomized | 74 | Tobramycin 300mg/12h | 4 months | 100% PA | - ↓Number of colonies (35% eradication) | – | Cough, bronchospasm, dyspnea |
| Placebo | Improved symptoms | |||||||
| Couch (2001)45 | Randomized | 74 | Tobramycin 300mg/12h | 4 weeks | 100% PA | - ↓Number of colonies (36% eradication) | 8% | Dyspnea, wheezing and thoracic discomfort |
| Placebo | Improved symptoms | |||||||
| Drobnic (2005)43 | Randomized | 30 | Tobramycin 300mg/12h | 6 months | 100% PA | - ↓Hospitalizations | No | Bronchospasm (10%) |
| Placebo | - ↓Length of hospitalizations | |||||||
| - ↓Density of colonies | ||||||||
| Scheinberg (2005)42 | Nonrandomized | 41 | Tobramycin 300mg/12h | 3 cycles14 days on and 3 cycles off | 100% PA | - Improved symptoms and HRQL | 5% | Cough, bronchospasm |
| - 22% Eradication PA | ||||||||
| Steinfort (2007)47 | Nonrandomized | 18 | Colistin 30mg (14 bronch. plus 4 COPD) | 41 months | 78% PA | -Improved HRQL | No | No |
| - ↓Loss in lung frequency | ||||||||
| Dhar (2010)46 | Nonrandomized (retrospective) | 19 | Colistin 1–2ml/12h (jet nebulizer) | 23.6 months | 100% PA | - ↓Volume and sputum colonies | – | No |
| - ↓Exacerbations and hospitalizations |
PA; Pseudomonas aeruginosa; COPD: chronic obstructive pulmonary disease; HRQL: health-related quality of life.
The side effects are usually minor and appear locally. The most frequent is bronchospasm (usually mild and reversible), dyspnea, cough and thoracic discomfort. Hemoptysis and tinnitus are less frequent and systemic adverse effects are very infrequent, although cases of ototoxicity and nephrotoxicity have been published. Pre-treatment with short-acting bronchodilators and respiratory physiotherapy are recommended before nebulization. They should not be used during exacerbations and it is necessary to take extreme precautions in patients with active hemoptysis, important bronchial hyperreactivity, auditory or renal problems and neuromuscular diseases. Therefore, it is recommended for the first dose to be administered at the hospital. Both the active ingredient itself as well as the preservation solution can cause side effects, especially bronchospasm. In some cases, the use of new nebulizers could generate greater bronchial hyperreactivity due to the increase in the flow of particles that they generate.51–54 As for the use of inhaled antibiotics in patients with chronic bronchial colonization/infection by other microorganisms other than PA, there is very little existing literature; therefore, treatment should be individualized.21
The future of inhaled antibiotic therapy seems very promising, not only for the treatment of chronic bronchial colonization-infection in patients with bronchiectasis but also for other types of infectious airway diseases. When we consulted the Clinicaltrials.gov database,55 at least 42 clinical assays are identified that are either being done or have recently concluded about the use of inhaled antibiotics for the treatment of pulmonary diseases, such as aztreonam (Cayston®, soon to be on the market), liposomal amikacin (Arikace®), gentamicin,56 liposomal and non-liposomal ciprofloxacin, vancomycin, a combination of tobramycin and phosphomycin, levofloxacin (Aeroquin®), tobramycin [TIP] and colistin in dry powdered form (Colobreath®). On the other hand, there is also important research on different methods of administration of these drugs that are achieving greater lung deposits and fewer side effects. Among these are: liposomal forms, in which the antibiotic is encapsulated in an aquatic environment surrounded by a lipid layer that are being used for the vehiculization of ciprofloxacin and amikacin57; the use of dry powder, which will soon be on the market for the use of tobramycin and colistin,58,59 ensuring more comfort for the patient by reducing the inhalation time without significantly modifying the lung deposit; and the improvement of the new mesh nebulizers. Some authors have observed that the lung deposit of inhaled ciprofloxacin in patients with bronchiectasis is more than 20% and that, with a single inhalation per day, local concentrations of the drug are reached that are 100 times greater than the minimum inhibitory concentration for some PPM, without causing any important adverse effects.60,61
Etiological TreatmentInitially, an aspect to always keep in mind in patients with bronchiectasis (with or without bacterial colonization) is the treatment of the baseline disease that generates it, if known. In this direction, both national as well as international guidelines21,22 clearly support ordering all the complementary studies necessary in order to determine the etiology of the bronchiectasis, especially in potentially curable or treatable diseases, with the intention of slowing down the loss of lung function. Thus, when given a patient with bronchiectasis, it is especially important to rule out the presence of antibody production deficiency, allergic bronchopulmonary aspergillosis, gastroesophageal reflux, obstruction of the bronchial tree, asthma or chronic obstructive pulmonary disease (COPD) (with or without alpha 1-antitripsin deficiency), infection by mycobacteria, CF and associated systemic diseases.
Anti-inflammatory TreatmentMacrolidesMacrolides present a series of immunomodulatory effects demonstrated in vitro and in vivo, regardless of the antibacterial qualities that they may have. Their effectiveness in bronchiectasis and in other respiratory diseases is mainly explained by their effect on bacterial virulence62,63 and inflammation.64 The prolonged administration of macrolides has been shown to be effective in bronchiectasis secondary to diffuse panbronchiolitis65 and secondary to CF, especially in patients with chronic bronchial infection by PA. In this group of patients, it has been observed that prolonged treatment with azithromycin reduces the number of exacerbations and improves lung function.66 In patients with CF without chronic bronchial infection by PA, azithromycin reduced the number of exacerbations but it has not been demonstrated to improve lung function.67,68
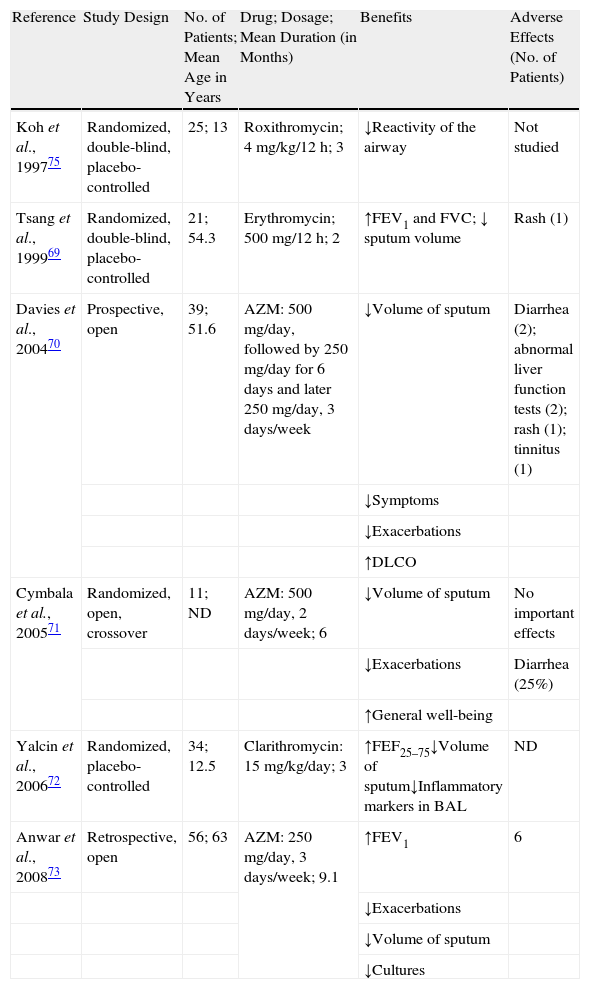
Several studies have researched the clinical and microbiological effects of macrolides in patients with non-CF bronchiectasis (Table 3). In 1999, Tsang et al.69 performed a randomized, double-blind study with 21 adult patients with bronchiectasis, comparing 8 weeks of treatment with erythromycin (500mg/12h) with a placebo. In this study, 76% of the patients were chronically colonized by PA. Compared with the placebo group, the patients treated with erythromycin presented a significant increase in FEV1 and FVC and a significant reduction in sputum volume. Treatment with erythromycin did not translate into a reduction in bacterial density or sputum inflammatory markers. In another open study, 33 patients who had presented at least 4 exacerbations during the previous year were treated with azithromycin at a dosage of 500mg, 3 times a week for at least 4 months.70 The authors observed a significant improvement in the symptoms and a reduction in the chronic colonization and in the frequency of exacerbations. In 2005, Cymbala et al.71 evaluated the effect of treatment with azithromycin for 6 months. The authors observed a decrease in the volume of sputum and in the frequency of exacerbations and an improvement in the general state of the patients. One year later, Yalcin et al.72 published a placebo-controlled, randomized study with clarithromycin in 34 children with bronchiectasis. The patients received clarithromycin at doses of 15mg/kg/day or placebo. The patients treated presented a decrease in the volume of sputum, with no significant changes in the lung function between the treated and placebo groups. In the bronchoalveolar lavage, there were observed reductions in the total number of leukocytes and the percentage of neutrophils, macrophages and interleukin (IL) 8. There were no significant changes in other inflammatory parameters studied, such as tumor necrosis factor or IL-10.
Studies Done With Macrolides in Patients With Non-Cystic Fibrosis Bronchiectasis.
| Reference | Study Design | No. of Patients; Mean Age in Years | Drug; Dosage; Mean Duration (in Months) | Benefits | Adverse Effects (No. of Patients) |
| Koh et al., 199775 | Randomized, double-blind, placebo-controlled | 25; 13 | Roxithromycin; 4mg/kg/12h; 3 | ↓Reactivity of the airway | Not studied |
| Tsang et al., 199969 | Randomized, double-blind, placebo-controlled | 21; 54.3 | Erythromycin; 500mg/12h; 2 | ↑FEV1 and FVC; ↓ sputum volume | Rash (1) |
| Davies et al., 200470 | Prospective, open | 39; 51.6 | AZM: 500mg/day, followed by 250mg/day for 6 days and later 250mg/day, 3 days/week | ↓Volume of sputum | Diarrhea (2); abnormal liver function tests (2); rash (1); tinnitus (1) |
| ↓Symptoms | |||||
| ↓Exacerbations | |||||
| ↑DLCO | |||||
| Cymbala et al., 200571 | Randomized, open, crossover | 11; ND | AZM: 500mg/day, 2 days/week; 6 | ↓Volume of sputum | No important effects |
| ↓Exacerbations | Diarrhea (25%) | ||||
| ↑General well-being | |||||
| Yalcin et al., 200672 | Randomized, placebo-controlled | 34; 12.5 | Clarithromycin: 15mg/kg/day; 3 | ↑FEF25–75↓Volume of sputum↓Inflammatory markers in BAL | ND |
| Anwar et al., 200873 | Retrospective, open | 56; 63 | AZM: 250mg/day, 3 days/week; 9.1 | ↑FEV1 | 6 |
| ↓Exacerbations | |||||
| ↓Volume of sputum | |||||
| ↓Cultures |
AZM: azithromycin; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; DLCO: carbon monoxide diffusion capacity; ND: no data; BAL: bronchoalveolar lavage; FEF25–75: forced expiratory flow between 25% and 75% of forced vital capacity.
Anwar et al.73 recently published the results of a retrospective study about the effects of azithromycin at a dose of 250mg, 3 times a week, in patients that had presented 3 or more exacerbations for 6 months. The patients had 50% less exacerbations, a reduction in the sputum volume and bacterial cultures and a mild increase in lung function. It is important to highlight that the majority of the isolations were of Haemophilus influenzae and Streptococcus pneumoniae, microorganisms usually sensitive to macrolides (unlike PA), therefore it is difficult to know if the improvement was due only to the immunomodulatory effects of the azithromycin or to its anti-microbial effects on these microorganisms. In the latest paper published to date, the authors arrive at the conclusion that erythromycin, at a dose of 250mg/day in adult patients with non-CF bronchiectasis, reduces the number of exacerbations and the consumption of antibiotics.74
In short, although more studies are needed to clearly understand the role of macrolides in the treatment of patients with non-CF bronchiectasis, there is some evidence that their use, especially that of azithromycin, can benefit patients with bronchiectasis who present frequent exacerbations.75 Their administration is recommended in chronic bronchial infection by PA or other microorganisms if the control of the symptoms is difficult despite adequate treatment.21 Although the optimal dosage (duration, dose, periodicity) has still not been clearly established, the dosage of azithromycin that is usually used is 250–500mg every 24h, depending on weight (patients >40kg: 500mg, and in patients <40kg: 250mg), 3 days per week, preferably on non-consecutive days. No studies have been done to demonstrate either effectiveness or safety in treatments of more than 12 months. A reasonable option could be to try out a treatment for 3 or 6 months and see the results in terms of quality of life, number of exacerbations, etc. If the results are not adequate, the treatment should be suspended. If not, it should be continued, carefully evaluating the risk/benefit ratio and watching for the possible appearance of secondary effects.
Both before initiating the treatment and then every 6 months, respiratory infection by non-tuberculous mycobacteria should be ruled out by means of a sputum analysis, as the patients with isolation of non-tuberculous mycobacteria should not receive monotherapy with macrolides due to the risk of increasing the selection of non-tuberculous mycobacteria strains resistant to macrolides. The most frequent secondary effects are gastrointestinal (nausea, diarrhea), elevated transaminases, reduced auditory capacity, as well as urogenital candidiasis, especially in women. Thus, periodic controls of the transaminases are recommended in the first few weeks of treatment and then every 6 months. In order to reduce as much as possible the gastrointestinal effects, it may be recommendable to administer oral probiotics for maintenance. It has been demonstrated that prolonged treatment with macrolides increases the resistances of the microorganisms present at the bronchial level (S. aureus, H. influenzae), which should be evaluated in future studies. Prolonged treatment with other anti-inflammatory drugs, such as oral corticosteroids or ibuprofen, is not recommended in non-CF bronchiectasis due to their secondary effects.21
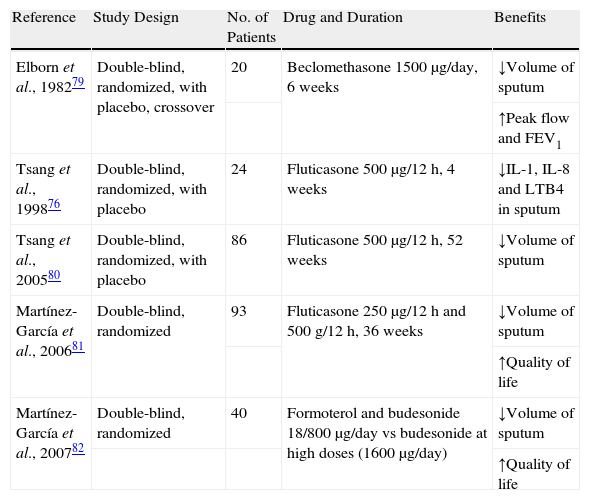
Inhaled CorticosteroidsInhaled corticosteroids can reduce inflammation and improve airway obstruction. Physiopathologically, they reduce the proinflammatory markers in sputum.76 There is not sufficient evidence to be able to recommend their routine use in stable patients, but their use could be assessed in adult patients with difficult-to-control symptoms,21,77 although special precautions must be taken when using high doses.78 A randomized, double-blind, placebo-controlled, crossover study done in adult patients with bronchiectasis79 showed a reduction of 18% in the production of sputum with a small improvement of FEV1 and the maximal expiratory flow that, although significant, had doubtful clinical significance. A study done years later demonstrated that the use of inhaled fluticasone at high doses (1000μg/day) is able to reduce the density of leukocytes and the inflammatory parameters in the sputum of patients with bronchiectasis, reducing the volume of expectoration and improving the quality of life of the patients.80 The increase in adverse effects when high doses are used mean that they are not generally recommended in patients with bronchiectasis, but instead in patients with greater bronchorrhea or airflow obstruction.81 Lastly, Martínez-García et al. observed for the first time in patients with bronchiectasis that the addition of a long-acting beta-2 adrenergic (formoterol) allows for inhaled corticoids to be reduced to half the dose, improving the clinical parameters and quality of life of the patients, with a reduction of local side effects.82Table 4 compiles the most significant studies in this regard.
Studies Done With Inhaled Corticosteroids in Patients With Non-Cystic Fibrosis Bronchiectasis.
| Reference | Study Design | No. of Patients | Drug and Duration | Benefits |
| Elborn et al., 198279 | Double-blind, randomized, with placebo, crossover | 20 | Beclomethasone 1500μg/day, 6 weeks | ↓Volume of sputum |
| ↑Peak flow and FEV1 | ||||
| Tsang et al., 199876 | Double-blind, randomized, with placebo | 24 | Fluticasone 500μg/12h, 4 weeks | ↓IL-1, IL-8 and LTB4 in sputum |
| Tsang et al., 200580 | Double-blind, randomized, with placebo | 86 | Fluticasone 500μg/12h, 52 weeks | ↓Volume of sputum |
| Martínez-García et al., 200681 | Double-blind, randomized | 93 | Fluticasone 250μg/12h and 500g/12h, 36 weeks | ↓Volume of sputum |
| ↑Quality of life | ||||
| Martínez-García et al., 200782 | Double-blind, randomized | 40 | Formoterol and budesonide 18/800μg/day vs budesonide at high doses (1600μg/day) | ↓Volume of sputum |
| ↑Quality of life |
FEV1: forced expiratory volume in one second; IL: interleukin; LT: leukotriene.
As there are no studies that defend the effectiveness and safety of prolonged treatment with oral corticosteroids or ibuprofen, their use is not recommended.21,83 Leukotriene receptor antagonists could be potentially useful in bronchiectasis as they inhibit the neutrophilic inflammation in the airways. However, there are no controlled studies to date that support such a practice in this pathology.84
Bronchodilator TreatmentThe mechanism of bronchial obstruction in bronchiectasis not associated with CF is not clear. It could be explained by various factors, such as the excessive production of mucus, the distortion of the bronchial architecture and the constriction of the smooth muscle of the airways. But, as bronchiectasis can coexist with asthma as well as with COPD, it is difficult to differentiate in studies when the obstruction of the airways is due to underlying asthma, COPD, bronchiectasis or a combination of these pathologies. Although in these patients it is frequent to observe an increase in bronchial hyperreactivity85,86 as well as a certain degree of reversibility of the bronchial obstruction with the use of inhaled bronchodilators,36,86–88 to date no randomized studies have been published that have adequately evaluated the role of the bronchodilators in bronchiectasis with prolonged treatment.88,89 In a placebo-controlled study, a greater increase in FVC and FEV1 was observed after salbutamol.90 There is no evidence for using inhaled anticholinergics in children with bronchiectasis.83 These drugs, however, can be effective in some adult patients.91 In general, it is recommended to assess the reversibility of the patient airway obstruction with salbutamol and ipratropium bromide and initiate treatment when an improvement in the lung function or symptoms is observed. The administration of inhaled bronchodilators is also recommended before physiotherapy or aerosolized antibiotics to prevent possible bronchospasms.21
Long-acting bronchodilators have a clear role in the management of the obstruction in asthma patients because they allow for reduced inhaled corticosteroid use and also lower the frequency of exacerbations. Thus, they could theoretically play a role in the treatment of patients in whom bronchiectasis coexists with asthma, although to date there is no good evidence upholding this practice in patients with bronchiectasis without asthma.92
Currently, there is no evidence that supports the use of methylxanthines in the treatment of patients with bronchiectasis,93 therefore its use is not recommended.
Respiratory RehabilitationThe objective of respiratory rehabilitation is to help mobilize secretions, improve ventilatory capacity, improve tolerance to exercise and reduce the dyspnea of the patients. There are devices that mechanically permeabilize the airways adequately, favoring the expulsion of the bronchial secretions and avoiding their accumulation and possible complications.94–97
Physical ExercisePhysical aerobic exercise improves physical tolerance and health-related quality of life. It is recommended that all patients perform moderate-to-intense exercise for 30min a day, 3 or 4 times a week or, if not, moderate physical activity every day, in addition to physiotherapy techniques.98–100
Respiratory PhysiotherapyThe objective of respiratory physiotherapy is to favor mucociliary clearance and to reduce the frequency of cough. Although there is no clear evidence that indicates which patients should benefit from physiotherapy techniques,101,102 it is a fact that is widely recognized by professionals who treat this pathology that the routine clearance of bronchial hypersecretion is a fundamental component in the management of patients who have productive chronic cough or evidence of mucus plugging on CT. Although there is no evidence whether patients with non-productive cough could also benefit from physiotherapy techniques, the consensus of experts is that they should perform respiratory physiotherapy at least during exacerbations.22 Physiotherapy should be done three times a day, after bronchodilator treatment and before inhaled antibiotics.103 There are several respiratory physiotherapy techniques that can be used in patients with bronchiectasis, but although according to certain studies one technique may be more effective than another,104 in reality there is no clear evidence about which is more effective. Assisted techniques require the help of another person (physiotherapist or caretaker), but there are alternatives that the patient can do alone, which provide more independence in the management and control of the disease. The choice will depend on the age of the patient and his/her capability to perform the technique. In general, self-administered techniques are recommended for better compliance.
Mucolytics and Hyperosmolar AgentsThe effectiveness of mucolytics has not been clearly demonstrated in patients with bronchiectasis105 or in patients with other pulmonary pathologies.106–108 The Cochrane systematic review,105 based on the paper by Olivieri et al.,109 suggests that bromhexine is the only mucolytic agent that has demonstrated a certain benefit in the treatment of exacerbations of patients with bronchiectasis.
The inhalation of hyperosmolar agents (hypertonic saline solution and mannitol in dry powder) is a much more promising therapy in patients with bronchiectasis.110 These agents favor the clearing of the airways in most respiratory diseases that are characterized by an excessive production of sputum, favoring the hydration of the airways and mucociliary clearance.111–113 The greatest evidence of the effectiveness of this type of agents has been demonstrated with the inhalation of a 7% hypertonic saline solution in patients with bronchiectasis secondary to CF.114 In these patients, it has been demonstrated that its inhalation reduces exacerbations, improves quality of life and slightly improves lung function.115 In patients with non-CF bronchiectasis, it has been demonstrated that 7% hypertonic saline solution can reduce the viscosity of the sputum and slightly improve lung function when compared with 0.9% saline solution.116 Although the inhalation of DNase was shown to be effective in CF, in bronchiectasis caused by other etiologies it may be ineffective117 or even harmful,118 therefore its use is not recommended.
Nutritional TreatmentPatients with evolved bronchiectasis usually present malnutrition, and there is a close relationship between malnutrition and lung function. All patients with bronchiectasis should receive nutritional education and control as part of their integral health care in order to maintain or achieve a normal nutritional state, either through natural nutrition and/or enteral nutrition, especially during exacerbations. Body mass index (BMI) should be one of the parameters controlled in the consultation of adult patients with bronchiectasis, and especially in those with severe disease,119,120 with the aim of early nutritional intervention when necessary. The administration of oral supplements should be considered in patients with a BMI <20kg/m2, or those with >20kg/m2 who are quickly losing weight (especially during exacerbations and hospitalizations).121
Treatment of ComplicationsThe most frequent complications of bronchiectasis are atelectasis, hemoptysis and respiratory failure.
AtelectasisLobar or segmental atelectasis can be due to the presence of intrabronchial mucus plugging or severe parenchymatous disease. Conventional treatment of atelectasis is based on the intensification of respiratory physiotherapy and on the administration of antibiotics, along with inhaled bronchodilators and even systemic corticosteroids. The administration of bronchodilators with aerosolized saline solution may be useful. If the conservative measures are not enough, bronchoscopy should be done to aspirate the thick secretions or mucus plug responsible for the atelectasis. If these mentioned measures fail, the need for lobectomy should be evaluated, although it must also be considered that this option may compromise any future lung transplantation.21
HemoptysisThis is one of the most frequent complications. It can range from very mild to very severe. This latter case is less frequent, although potentially mortal. The most frequent cause of hemoptysis is an exacerbation. There are few publications about the management of hemoptysis in adults with non-CF bronchiectasis. In these patients, the threat of hemoptysis requires, in addition to the standard measures for all hemoptysis (maintain the airway free, optimize oxygenation and stabilize the patient hemodynamically), the administration of intravenous antibiotics, avoidance of nebulized drugs and physiotherapy for at least the first 24–48h, and embolization of the pathologic bronchial arteries of the area of the hemorrhage.122–125 Surgery is only indicated when there is vital risk, when the origin of the hemorrhage is well located and when the hemoptysis cannot be controlled with the previously mentioned measures.
Respiratory FailureRespiratory failure is the most frequent cause of death in patients with bronchiectasis. It appears in the severest forms of the disease or temporarily during exacerbations. Due to the fact that there are no specific studies analyzing how to manage these patients, the main general recommendations for oxygen treatment should be followed. Non-invasive mechanical ventilation can be used in patients in situation of overall respiratory failure, although the use of this treatment and the appearance of possible complication should be closely monitored.126–129
The great advances in antibiotic treatment over the last few decades have relegated the surgical treatment of bronchiectasis to only exceptional cases, such as the extraction of a tumor or a foreign body, localized bronchiectasis with frequent recurrent infections that do not respond to pharmaceutical treatment, the causes of severe hemoptysis in which the embolization of bronchial arteries is not effective, suspicion of resistant microorganisms (such as non-tuberculosis mycobacteria) or abscessed bronchiectasis that is not curable with antibiotic treatment.130–132
Conflicts of InterestAuthors have no conflict of interests to declare.
Please cite this article as: Martínez García MÁ, et al. Tratamiento de las bronquiectasias no debidas a fibrosis quística. Arch Bronconeumol. 2011. doi:10.1016/j.arbres.2011.06.003.