Among the extrapulmonary manifestations of COPD, dysfunction and loss of muscle mass/weight are those that have the greatest impact on the quality of life of patients. Our objective was to evaluate the molecular mechanisms that are potentially implicated in the limited development of muscle mass in the diaphragm and gastrocnemius of mice with experimentally induced emphysema.
MethodsAn experimental model in mice, in which emphysema was induced by means of the local instillation of elastase (n=6), while saline was administered to the controls (n=7). We determined the levels of oxidative stress, proteolytic systems, signalling pathways, growth factors and cell differentiation (Western blot) in the diaphragm and gastrocnemius of all the mice after 34 weeks.
ResultsUpon comparing the mice with emphysema with the controls, the following findings were observed: (1) lower total body weight and lower weight of the diaphragm and gastrocnemius; (2) in the diaphragm, the levels of protein oxidation were increased, the mitochondrial antioxidant systems reduced, the levels of myostatin and of the ERK1/2 and FoxO1 signalling pathways were higher, and the myosin content was lower (67%); and (3) in the gastrocnemius of the emphysematous mice, the cytosolic antioxidants were decreased and the levels of myostatin and of the JNK and NF-kB signalling pathways were increased.
ConclusionsThe reduction of the myosin content observed in the diaphragm of mice with emphysema could explain their smaller size. Oxidative stress, myostatin and FoxO could be implicated in the loss of this structural protein.
Entre las manifestaciones extrapulmonares de la EPOC, la disfunción y la pérdida de peso muscular son las de mayor repercusión en la calidad de vida de los pacientes. Nuestro objetivo fue evaluar los mecanismos moleculares potencialmente implicados en el menor desarrollo de masa muscular en el diafragma y gastrocnemio de ratones con enfisema inducido experimentalmente.
MétodosModelo experimental en ratones, a los que se les indujo un enfisema mediante instilación local de elastasa (n=6), administrándose suero fisiológico en los controles (n=7). Se determinaron los niveles de estrés oxidativo, sistemas de proteólisis, vías de señalización, factores de crecimiento y diferenciación celular (western-blot) en el diafragma y el gastrocnemio de todos los ratones tras 34 semanas.
ResultadosEn los ratones con enfisema respecto de los controles, se observaron los siguientes hallazgos: a) una menor ganancia de peso corporal total y un menor peso del diafragma y del gastrocnemio; b) en el diafragma, los niveles de oxidación proteica estaban aumentados, los sistemas antioxidantes mitocondriales disminuidos, los niveles de miostatina y los de las vías de señalización ERK1/2 y FoxO1 fueron superiores, y el contenido de miosina fue menor (67%), y c) en el gastrocnemio de los ratones enfisematosos, los antioxidantes citosólicos estaban disminuidos, y los niveles de miostatina y los de las vías de señalización JNK y NF-kB estaban incrementados.
ConclusionesLa reducción del contenido en miosina observado en el diafragma de ratones con enfisema podría explicar su menor tamaño. El estrés oxidativo, la miostatina y FoxO podrían estar implicados en la pérdida de esta proteína estructural.
Muscle dysfunction, whether or not accompanied by loss of muscle mass, is one of the major systemic manifestations of chronic obstructive pulmonary disease (COPD), and it seriously impacts the patient's quality of life.1 Although the aetiology of this muscle dysfunction has not yet been well explained, various mechanisms, such as immobilization, hypoxia, systemic inflammation, and oxidative stress, appear to contribute to a greater or lesser degree.2 It should be pointed out that elevated oxidative stress levels in respiratory and peripheral muscles have been shown to be a characteristic feature of patients with COPD,3–8 and this could also act as an induction mechanism for loss of muscle mass and muscle atrophy.9 From a molecular standpoint, this translates to reduced protein synthesis along with increased activity in muscle protein degradation systems in different processes and diseases, such as cancerous cachexia and COPD.10,11
Various molecular mechanisms contribute to protein degradation in mammalian muscle, such as lysosomes, autophagy, calpains, caspase 3, and the ubiquitin–proteasome system. Different studies have demonstrated that this last proteolytic system plays a leading role in muscle protein degradation in processes that are extremely common, such as cancerous cachexia and COPD.11 With regard to the signalling pathways involved in increased protein catabolism processes, it has been demonstrated recently that the forkhead box O (FoxO) family of transcription factors regulates expression of atrogin-1 in the quadriceps of patients with COPD12 and in the diaphragm of mechanically ventilated patients.13 Whether other signalling pathways, especially those sensitive to oxidants, could also be involved in regulating muscle proteolysis in chronic processes like COPD remains to be clarified, however. Another aspect still remaining to be clarified is identification of the muscle proteins primarily susceptible to degradation in the muscles of patients experiencing processes that involve the loss of muscle mass. It would be interesting to determine whether the proteins that, in previous studies,4,7,8 have shown a heightened susceptibility to oxidant effects would also be the main ones degraded by the proteolytic systems in muscle. Another aspect of major interest—and still to be determined—is whether the respiratory muscles and peripheral muscles have expression patterns in common for the different markers of proteolysis.
Our hypothesis was to identify molecular mechanisms that may be involved in the lesser development of muscle mass in respiratory and peripheral muscles in animals with a major chronic lung disease such as emphysema. Thus, a first objective of this study was to determine various markers of oxidative stress, proteolytic systems in muscle—among them, myostatin—signalling pathways, and muscle proteins susceptible to degradation (actin, myosin, and creatine kinase) in the diaphragm and gastrocnemius of mice with emphysema induced experimentally by administration of elastase. Another more secondary objective of the study was to establish an animal model of emphysema with respiratory and peripheral muscle involvement with which studies based on different objectives could be carried out in the future and various therapeutic strategies could be evaluated. In view of the results obtained in our research, it may be concluded that the experimental animal model also answers to this second objective.
MethodsStudy Population and Experimental GroupsSo as to avoid problems related to aging, the study used young adult male mice (age 2 months, body weight 21–23g) from the A/J strain, in whom emphysema was induced, along with control mice. We used a classic model of pulmonary emphysema through a single instillation (oropharyngeal aspiration) of elastase-high purity (EC134GI, Elastin Products Company), with a concentration of 0.15mg/100g of weight, or the equivalent of 20U of enzyme per 100g of weight. This is a methodology previously validated in other studies14,15 and in which the different stages of alveolar damage have been properly characterized. At the beginning of the study, the mice were randomly assigned to 2 groups: 1) mice with emphysema, slaughtered at 34 weeks into their disease (No.=6) and 2) control group, with only normal saline instilled into the oropharyngeal cavity and also slaughtered at 34 weeks following this single instillation (No.=7). Throughout the 34-week study period, the animals in both groups received daily nourishment and water ad libitum and remained under routine housing environment conditions. They also maintained a physical activity level typical for this type of animal throughout the course of the study.
This was a controlled study designed in accordance with our institution's current regulations on animal experimentation and with the Helsinki convention on the use and care of animals. The stipulations of current laws regulating the protection of the animals used for experimentation and other scientific purposes (Real Decreto [Royal Decree] 1201/2005 of 10 October) were also taken into account. All experiments were approved by the Experimentation Ethics Committee of the Centro para la Investigación Médica Aplicada (CIMA) [Centre for Applied Medical Research] in Pamplona (Navarra). It should be mentioned that, for reasons of an ethical nature, the mice used in this research had also been used in a previous study,15 the objective of which was to quantify the degree of emphysema using various methodologies.
Characteristics of the AnimalsHistological confirmation of the presence of emphysema in the lungs of the diseased mice, compared to the control animals, was quantitatively completed in a previous study.15 In the same study, lung distensibility was also evaluated in both groups of mice prior to slaughtering them, and the corresponding data were published.15
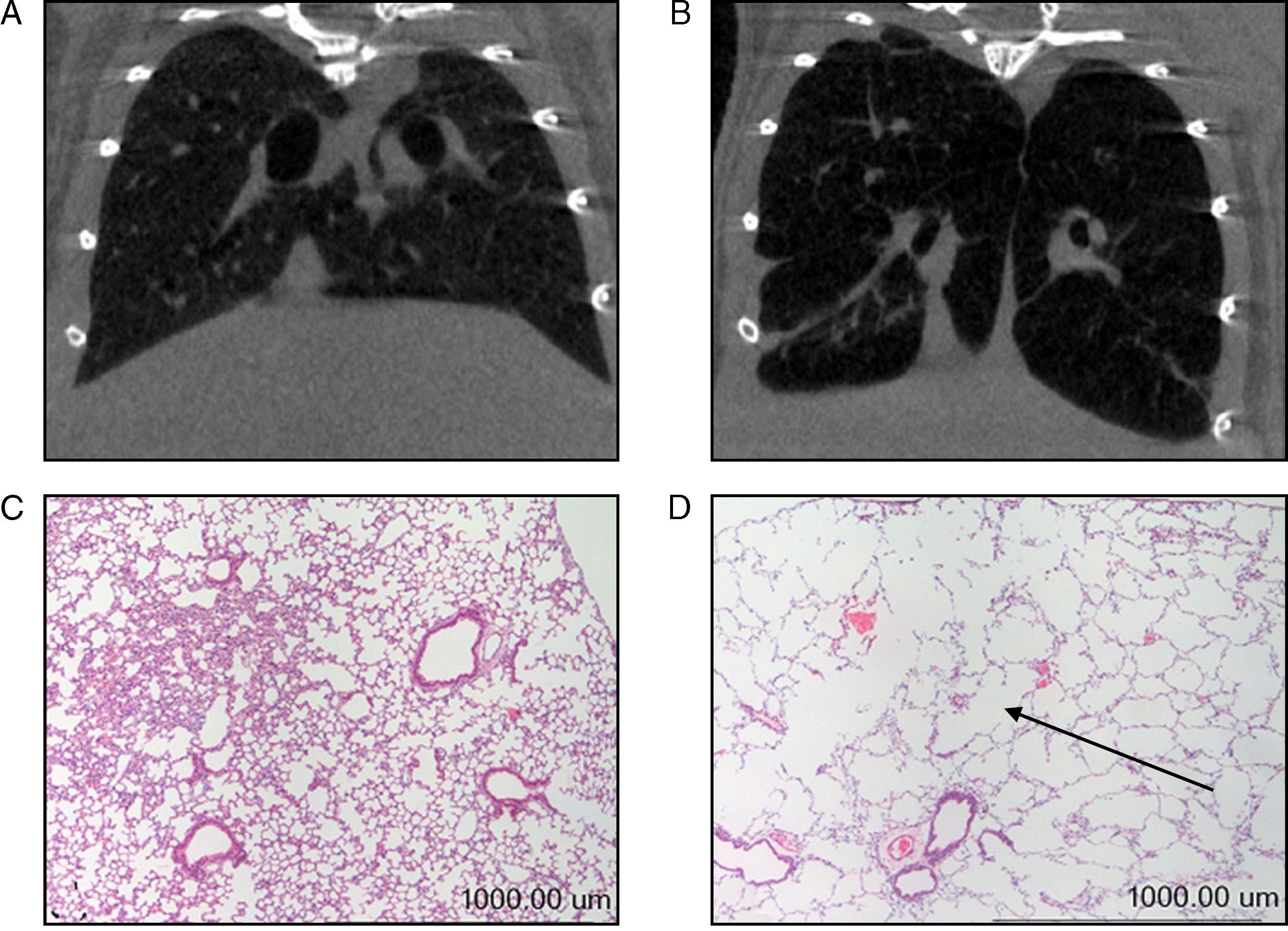
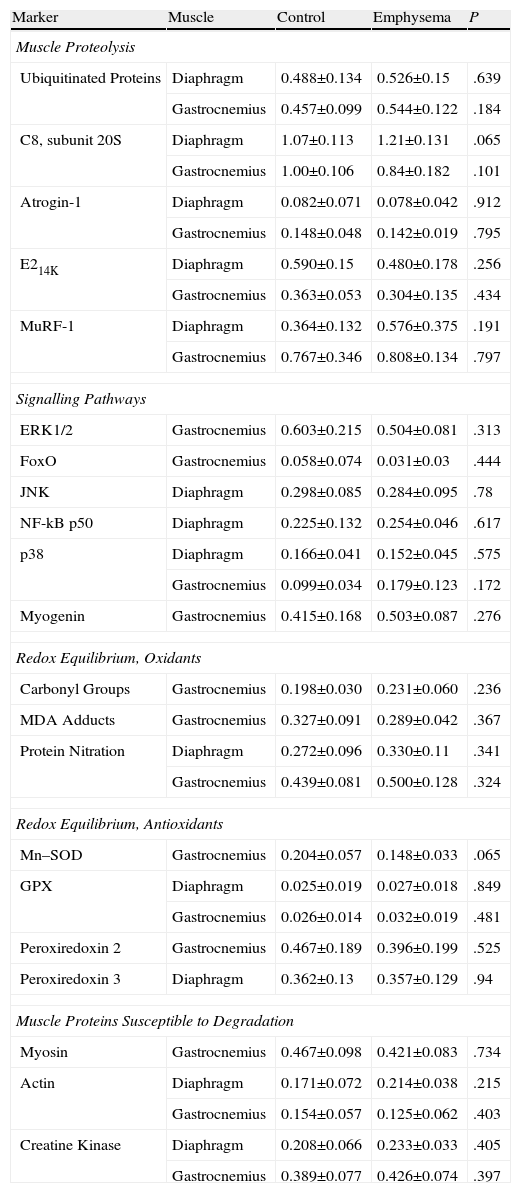
In our study, prior to their slaughter (week 34), computerized axial tomography (CAT scan) was also done on the 2 groups of mice for radiographic confirmation of the presence of emphysema in the diseased animals (Fig. 1A–B). All animals were weighed on day 0 and immediately prior to their slaughter (week 34). Given the animals’ age, they all had a tendency to gain weight over the course of the study. The percent gain in body weight for each animal, from day 0 (baseline weight) to week 34, was used as a study variable in all the mice.
(A) Computerized axial tomography of control mouse lung. (B) Computerized axial tomography of lung of mouse treated with elastase. (C) Haematoxylin and eosin stain of control mouse lung. (D) Haematoxylin and eosin stain of lung of mouse treated with elastase. The arrow indicates an area of lung parenchyma in which increased interalveolar space is appreciated.
In the 2 groups of mice, the diaphragm, gastrocnemius muscle, and lungs were obtained. Prior to the slaughter, sodium pentobarbital 50mg/kg was administered intraperitoneally and, after confirming that the animal was completely anaesthetized and feeling no pain, these extractions were performed, beginning with the extremity muscle and ending with the diaphragm and lungs. Once the animal's muscles were extracted, it was immediately frozen with liquid nitrogen for subsequent preservation at −80°C. The extracted lungs were fixed in formalin at a constant pressure of 20cm H2O and then embedded in paraffin for making the appropriate histological sections and slices.15
Molecular Biology ExperimentsMorphological AnalysisAs mentioned above, the degree of emphysema in the mice was quantified in a previous study using various methodologies.15 Given that the presence of emphysema had already been clearly confirmed in the lungs of all animals used in our study,15 these assessments were not repeated in our study; however, new histological slices were taken of lungs from both groups of animals and stained with haematoxylin and eosin for macroscopic and qualitative evaluation of the increase of interalveolar spaces in the lungs of the mice with emphysema (Fig. 1C and D).
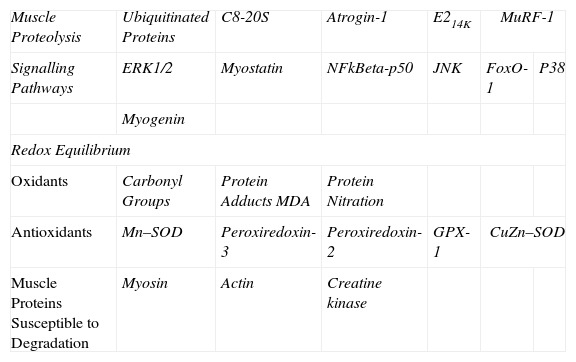
Identification of Molecular MarkersVarious molecular markers of proteolysis, signalling pathways, oxidative stress, and muscle proteins were determined in the diaphragm and gastrocnemius muscle for the 2 groups of animals using the Western blot technique, following to the letter the same procedures previously published by the group.3–5,7,8 In brief, the diaphragm and gastrocnemius specimens were homogenized in a lysis buffer, and the protein concentration was calculated using the Bradford method.16 The same amount of protein was loaded into each gel well for all specimens. The proteins underwent one-dimensional electrophoresis and were then transferred to a membrane and incubated with the following primary antibodies (Table 1):
- •
Oxyblot kit anti-carbonyl groups (Chemicon International Inc., Temecula, CA, USA);
- •
anti-nitrotyrosine (Invitrogen, Van Allen Way, Carlsbad, CA, USA);
- •
anti-malondialdehyde-protein adducts (MDA, Academy Bio-Medical Company, Inc., Houston, TX, USA);
- •
anti-CuZn-superoxide dismutase (SOD), anti-Mn-SOD, anti-creatine kinase, anti-mitogen activated kinase (MAPK), extracellular kinase (ERK)1/2, anti-forkhead box O (FoxO), anti-c-Jun terminal (JNK), anti-NF-kB p50, anti-MAPK p38, and anti-ligase atrogin-1 (Santa Cruz Biotechnology, CA, USA);
- •
anti-glutathione peroxidase-1, anti-peroxiredoxin-2, and anti-peroxiredoxin-3 (AB Frontier, Seoul, South Korea);
- •
anti-actin (Sigma-Aldrich, St. Louis, MO, USA);
- •
anti-myosin (Upstate, Billerica, MA, USA);
- •
anti-myostatin (Bethyl, Montgomery, TX, USA);
- •
anti-subunit C8 of the 20S proteasome complex (Biomol, Plymouth Meeting, PA, USA);
- •
anti-conjugating enzyme E214K and anti-ubiquitinated proteins (Boston Biochem, Cambridge, MA, USA); and
- •
anti-ligase MuRF-1 (muscle-specific ring finger-1) (Everest Biotech, Oxfordshire, United Kingdom).
Summary of All Markers Analysed in Mouse Muscles.
| Muscle Proteolysis | Ubiquitinated Proteins | C8-20S | Atrogin-1 | E214K | MuRF-1 | |
| Signalling Pathways | ERK1/2 | Myostatin | NFkBeta-p50 | JNK | FoxO-1 | P38 |
| Myogenin | ||||||
| Redox Equilibrium | ||||||
| Oxidants | Carbonyl Groups | Protein Adducts MDA | Protein Nitration | |||
| Antioxidants | Mn–SOD | Peroxiredoxin-3 | Peroxiredoxin-2 | GPX-1 | CuZn–SOD | |
| Muscle Proteins Susceptible to Degradation | Myosin | Actin | Creatine kinase | |||
E214k: ubiquitin-conjugating enzyme E2. ERK1/2: extracellular signal-regulated kinase 2. NF-kBeta p50: nuclear factor kappa beta subunit p50. JNK: c-Jun-amino-terminal kinase-interacting protein 3. FoxO: forkhead box protein O. MDA: malondialdehyde. Mn–SOD: manganese superoxide dismutase. GPX: glutathione peroxidase. CuZn–SOD: copper–zinc superoxide dismutase. Atrogin-1: muscle atrophy F-box protein. MuRF-1: muscle-specific RING finger protein 1.
The markers analysed in our study are summarized in Table 1. In each animal and muscle, the optical density for each parameter was calculated using the computer program Quantity One, version 4.6.5 (Bio-Rad Laboratories, Hercules, CA, USA). Once the electrophoresis was completed for each marker, Coomassie stain was used to ensure equal protein load in all the gel wells.17 In the case of markers of oxidative stress (carbonylated and nitrated proteins and MDA–protein adducts) and proteolysis (ubiquitinated proteins), the final value for each mouse muscle was obtained via the sum of the optical densities of all protein bands detected in each case.
Statistical AnalysisThe numerical variables (percent change in weight and optical densities for each marker) are expressed as mean and standard deviation for each group of mice. For each variable, the means for the 2 groups were compared using the t-test for independent samples. Statistical significance was set as a P value of <.05.
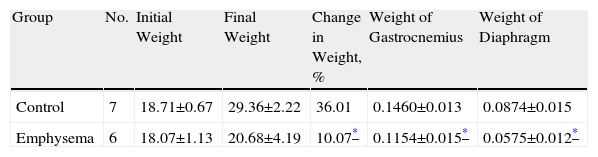
ResultsCharacteristics of the AnimalsIn our study, radiographic techniques (CAT scan) and haematoxylin and eosin staining (Fig. 1A–D, respectively) were used to qualitatively confirm the presence of emphysema in the mice treated with elastase, compared to the control animals. When the study period was over (week 34), total body weight gain for the emphysematous mice was significantly less than body weight gain for the control mice (Table 2). Likewise, the muscles examined in our study—diaphragm and gastrocnemius—also weighed significantly less in the emphysematous mice than in the control mice (Table 2).
Percent Change in Body Weight From Baseline to End of Study and Absolute Weight of Diaphragm and Gastrocnemius Muscles.
| Group | No. | Initial Weight | Final Weight | Change in Weight, % | Weight of Gastrocnemius | Weight of Diaphragm |
| Control | 7 | 18.71±0.67 | 29.36±2.22 | 36.01 | 0.1460±0.013 | 0.0874±0.015 |
| Emphysema | 6 | 18.07±1.13 | 20.68±4.19 | 10.07* | 0.1154±0.015* | 0.0575±0.012* |
No.: number of animals.
The data is presented as mean ± standard deviation.
No significant differences were observed in the diaphragm and gastrocnemius of the animals with emphysema, compared to the control animals, in terms of total levels of ubiquitinated protein, subunit C8 of the proteasome, ligase E2 atrogin-1, ubiquitin-conjugating enzyme E214k, or ligase E3 MuRF-1 (Table 3).
Molecular Markers Showing No Significant Difference in Protein Level Between the Two Groups of Animals.
| Marker | Muscle | Control | Emphysema | P |
| Muscle Proteolysis | ||||
| Ubiquitinated Proteins | Diaphragm | 0.488±0.134 | 0.526±0.15 | .639 |
| Gastrocnemius | 0.457±0.099 | 0.544±0.122 | .184 | |
| C8, subunit 20S | Diaphragm | 1.07±0.113 | 1.21±0.131 | .065 |
| Gastrocnemius | 1.00±0.106 | 0.84±0.182 | .101 | |
| Atrogin-1 | Diaphragm | 0.082±0.071 | 0.078±0.042 | .912 |
| Gastrocnemius | 0.148±0.048 | 0.142±0.019 | .795 | |
| E214K | Diaphragm | 0.590±0.15 | 0.480±0.178 | .256 |
| Gastrocnemius | 0.363±0.053 | 0.304±0.135 | .434 | |
| MuRF-1 | Diaphragm | 0.364±0.132 | 0.576±0.375 | .191 |
| Gastrocnemius | 0.767±0.346 | 0.808±0.134 | .797 | |
| Signalling Pathways | ||||
| ERK1/2 | Gastrocnemius | 0.603±0.215 | 0.504±0.081 | .313 |
| FoxO | Gastrocnemius | 0.058±0.074 | 0.031±0.03 | .444 |
| JNK | Diaphragm | 0.298±0.085 | 0.284±0.095 | .78 |
| NF-kB p50 | Diaphragm | 0.225±0.132 | 0.254±0.046 | .617 |
| p38 | Diaphragm | 0.166±0.041 | 0.152±0.045 | .575 |
| Gastrocnemius | 0.099±0.034 | 0.179±0.123 | .172 | |
| Myogenin | Gastrocnemius | 0.415±0.168 | 0.503±0.087 | .276 |
| Redox Equilibrium, Oxidants | ||||
| Carbonyl Groups | Gastrocnemius | 0.198±0.030 | 0.231±0.060 | .236 |
| MDA Adducts | Gastrocnemius | 0.327±0.091 | 0.289±0.042 | .367 |
| Protein Nitration | Diaphragm | 0.272±0.096 | 0.330±0.11 | .341 |
| Gastrocnemius | 0.439±0.081 | 0.500±0.128 | .324 | |
| Redox Equilibrium, Antioxidants | ||||
| Mn–SOD | Gastrocnemius | 0.204±0.057 | 0.148±0.033 | .065 |
| GPX | Diaphragm | 0.025±0.019 | 0.027±0.018 | .849 |
| Gastrocnemius | 0.026±0.014 | 0.032±0.019 | .481 | |
| Peroxiredoxin 2 | Gastrocnemius | 0.467±0.189 | 0.396±0.199 | .525 |
| Peroxiredoxin 3 | Diaphragm | 0.362±0.13 | 0.357±0.129 | .94 |
| Muscle Proteins Susceptible to Degradation | ||||
| Myosin | Gastrocnemius | 0.467±0.098 | 0.421±0.083 | .734 |
| Actin | Diaphragm | 0.171±0.072 | 0.214±0.038 | .215 |
| Gastrocnemius | 0.154±0.057 | 0.125±0.062 | .403 | |
| Creatine Kinase | Diaphragm | 0.208±0.066 | 0.233±0.033 | .405 |
| Gastrocnemius | 0.389±0.077 | 0.426±0.074 | .397 | |
The data is expressed as mean ± standard deviation, differences between the 2 groups; optical densities (OD) expressed in the form of arbitrary units (au).
E214k: ubiquitin-conjugating enzyme E2. ERK1/2: extracellular signal-regulated kinases. NF-kB p50: nuclear factor kappa beta subunit p50. JNK: c-Jun-amino-terminal kinase-interacting protein 3. FoxO: forkhead box protein O. MDA: malondialdehyde. Mn–SOD: manganese superoxide dismutase. GPX: glutathione peroxidase. CuZn–SOD: copper–zinc superoxide dismutase. Atrogin-1: muscle atrophy F-box protein. MuRF-1: muscle-specific RING finger protein 1.
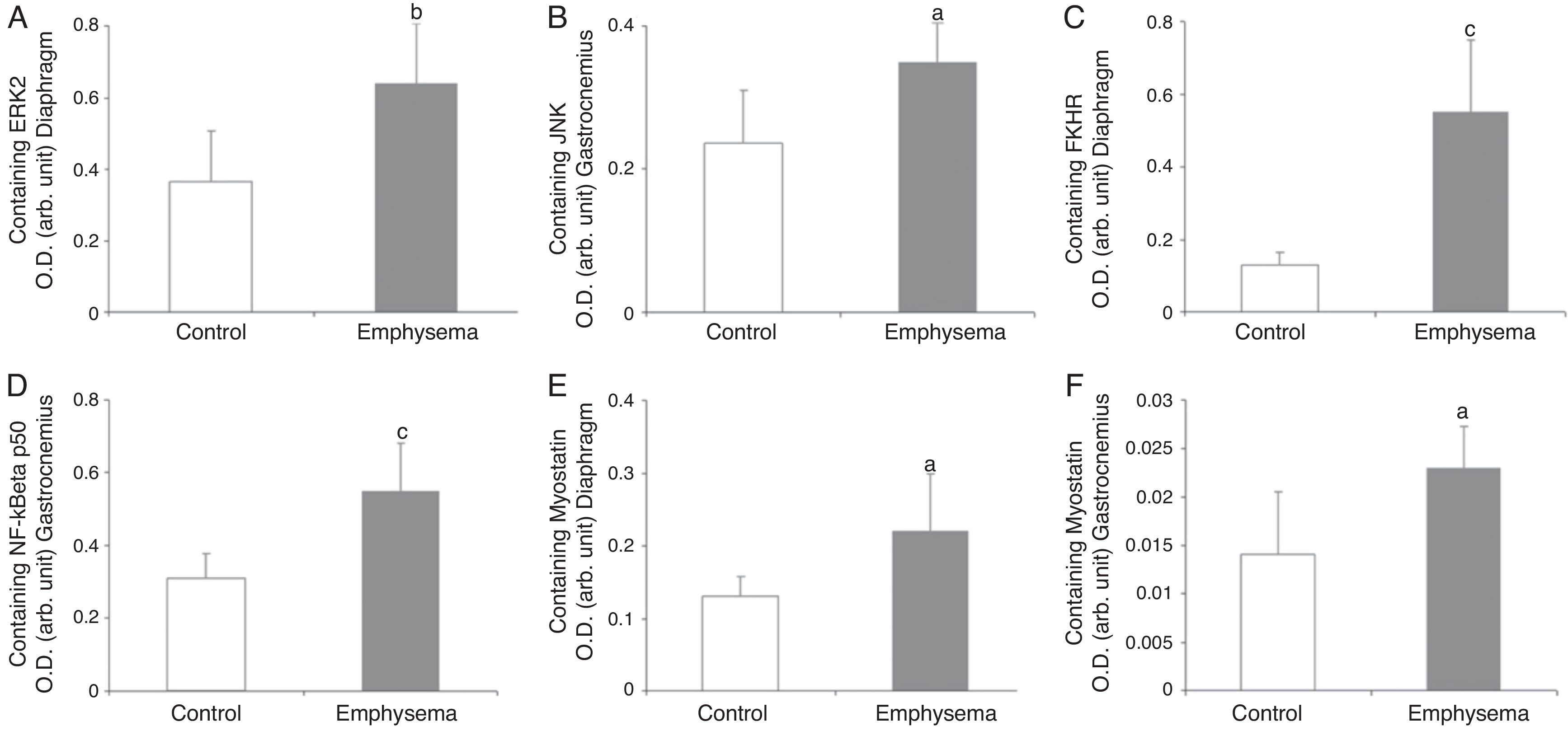
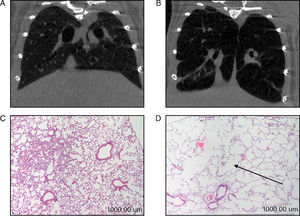
In the diaphragm (Fig. 2A) but not in the gastrocnemius (Table 3), a significant increase in enzyme ERK1/2 was observed in the animals with emphysema, compared to the controls. JNK pathway protein levels were higher in the gastrocnemius muscle of the mice with emphysema, compared to the controls (Fig. 2B), but this was not the case in the diaphragm (Table 3). No significant differences were detected between the mice with emphysema and the controls in any of the muscles with respect to marker p38 (Table 3).
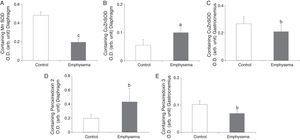
Protein content of markers in muscles of mice with emphysema and in the control animals. (A) Extracellular signal-regulated kinase 2 (ERK2) content of diaphragm muscle. (B) Forkhead box protein O (FKHR) content of diaphragm muscle. (C) c-Jun-amino-terminal kinase-interacting protein 3 (JNK) content of gastrocnemius muscle. (D) Nuclear factor kappa beta subunit p50 (NF-kBeta p50) content of gastrocnemius muscle. (E) Myostatin content of diaphragm muscle. (F) Myostatin content of gastrocnemius muscle. OD, optical density; au, arbitrary units. Mean values and standard deviation. aP<.05; bP<.01; cP<.001.
A significant increase in FoxO1 levels was observed in the diaphragm of the emphysematous mice, compared to the controls (Fig. 2C), but the same result was not seen in the gastrocnemius (Table 3).
NF-kB PathwayTranscription factor p50 levels were higher in the gastrocnemius of the emphysematous mice, compared to the controls (Fig. 2D), but not in the diaphragm (Table 3).
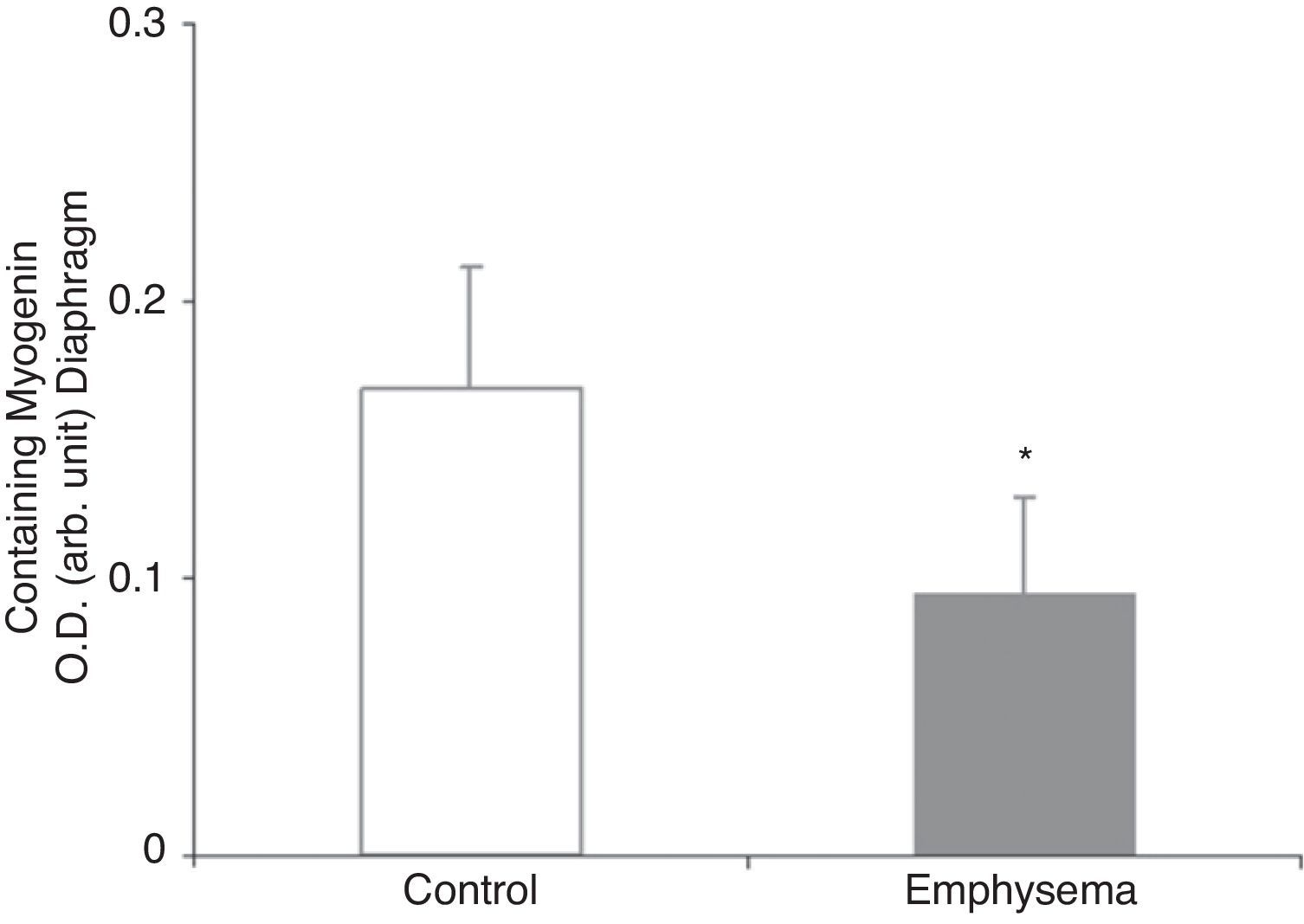
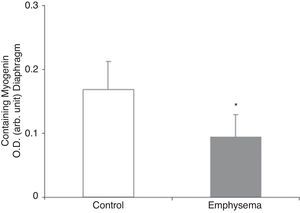
Muscle Growth and DifferentiationWhile myostatin protein levels were higher in the diaphragm and gastrocnemius of the mice with emphysema (Fig. 2E and F), myogenin transcription factor levels were lower only in the diaphragm (Fig. 3) and not in the gastrocnemius of these mice (Table 3).
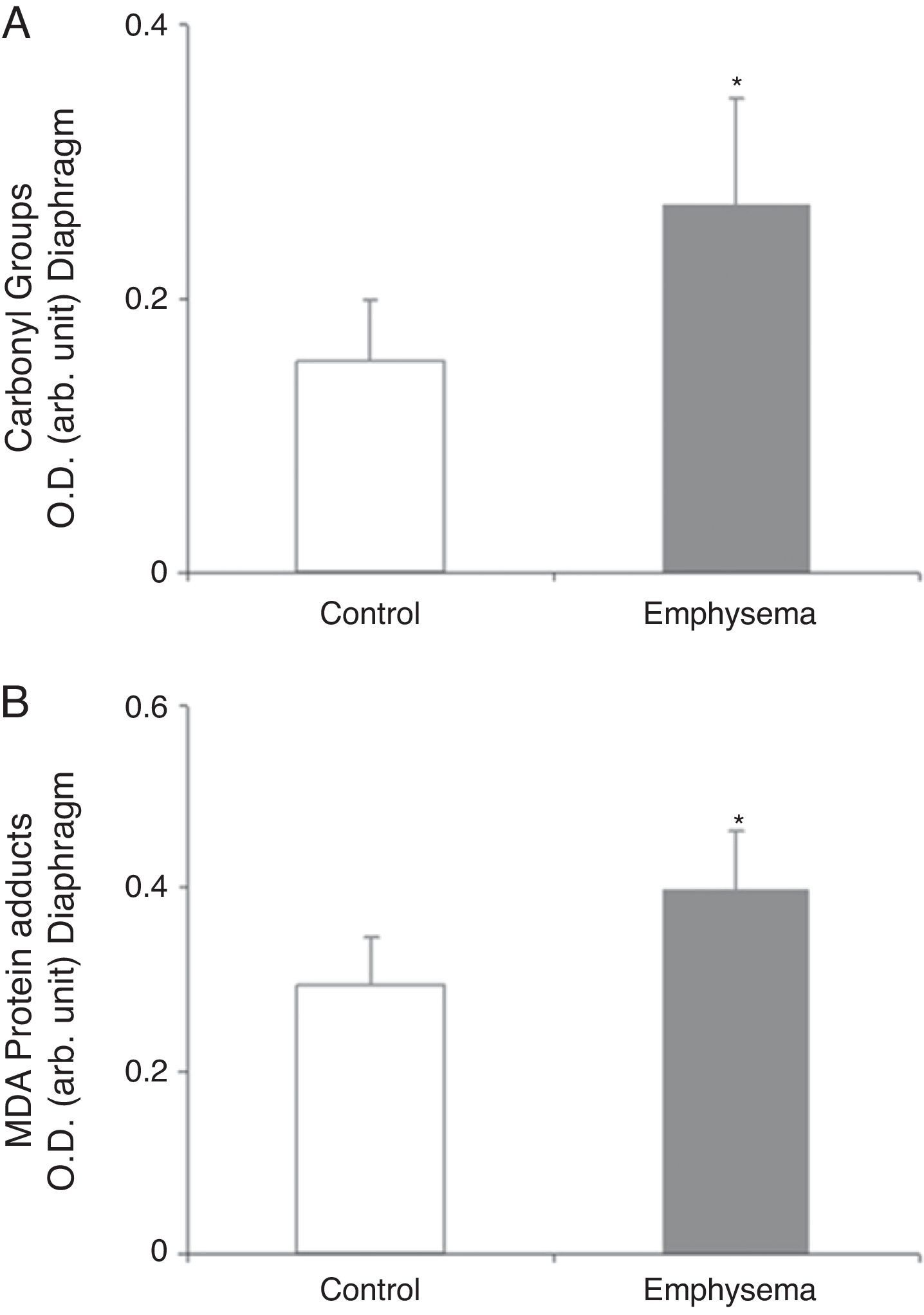
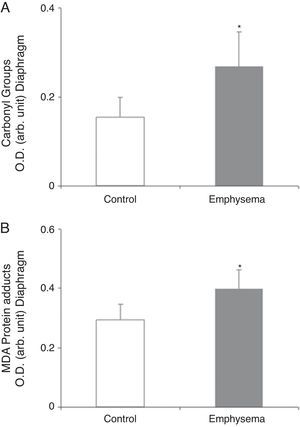
Redox EquilibriumProtein OxidationTotal carbonylated protein and MDA–protein adducts levels were higher in emphysematous mouse diaphragms than in those of the controls (Fig. 4A and B), while no differences were observed in these markers in the gastrocnemius for both groups of animals (Table 3).
Protein content of markers of oxidative stress in diaphragm of mice with emphysema and in the control animals. (A) Carbonyl group content of diaphragm muscle. (B) Malondialdehyde–protein adducts (MDA) content of diaphragm muscle. OD, optical density; au, arbitrary units. Mean values and standard deviation. *P<.01.
No significant differences were observed between the mice with emphysema and the controls in terms of nitrated protein levels in either diaphragm or gastrocnemius muscle (Table 3).
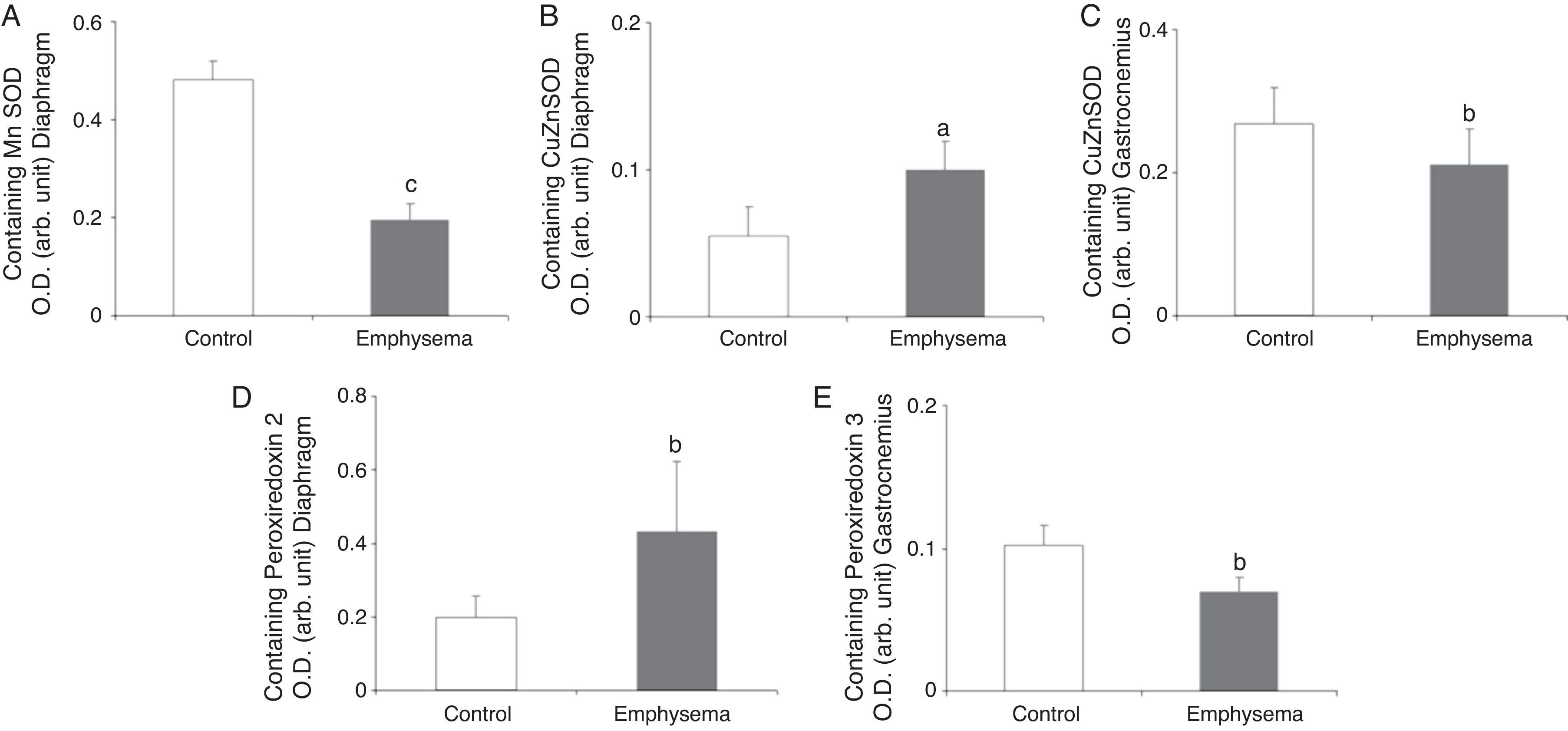
Antioxidants: Superoxide DismutasesA significant reduction in diaphragm Mn–SOD content was observed in the mice with emphysema, compared to the controls (Fig. 5A), but in the gastrocnemius, there were no differences between the groups (Table 3). CuZn–SOD protein levels were higher in the diaphragm of mice with emphysema, compared to the controls (Fig. 5B), while a significant reduction was observed in the gastrocnemius content of this enzyme (Fig. 5C).
Protein content of markers of oxidative stress in muscles of mice with emphysema and in the control animals. (A) Manganese superoxide dismutase (Mn–SOD) content of diaphragm muscle. (B) Copper–zinc superoxide dismutase (CuZn–SOD) content of diaphragm muscle. (C) Copper–zinc superoxide dismutase (CuZn–SOD) content of gastrocnemius muscle. (D) Peroxiredoxin-2 content of diaphragm muscle. (E) Peroxiredoxin-3 content of gastrocnemius muscle. OD, optical density; au, arbitrary units. Mean values and standard deviation. aP<.05; bP<.01; cP<.001.
A significant increase was observed in diaphragm peroxiredoxin-2 levels in the mice with emphysema, in comparison with the controls (Fig. 5D and Table 3). Peroxiredoxin-3 protein levels were lower in the gastrocnemius of the mice with emphysema (Fig. 5E) but not in the diaphragm (Table 3). In terms of glutathione peroxidase-1 protein levels, no significant differences were observed between the 2 groups of animals in either the diaphragm or the gastrocnemius (Table 3).
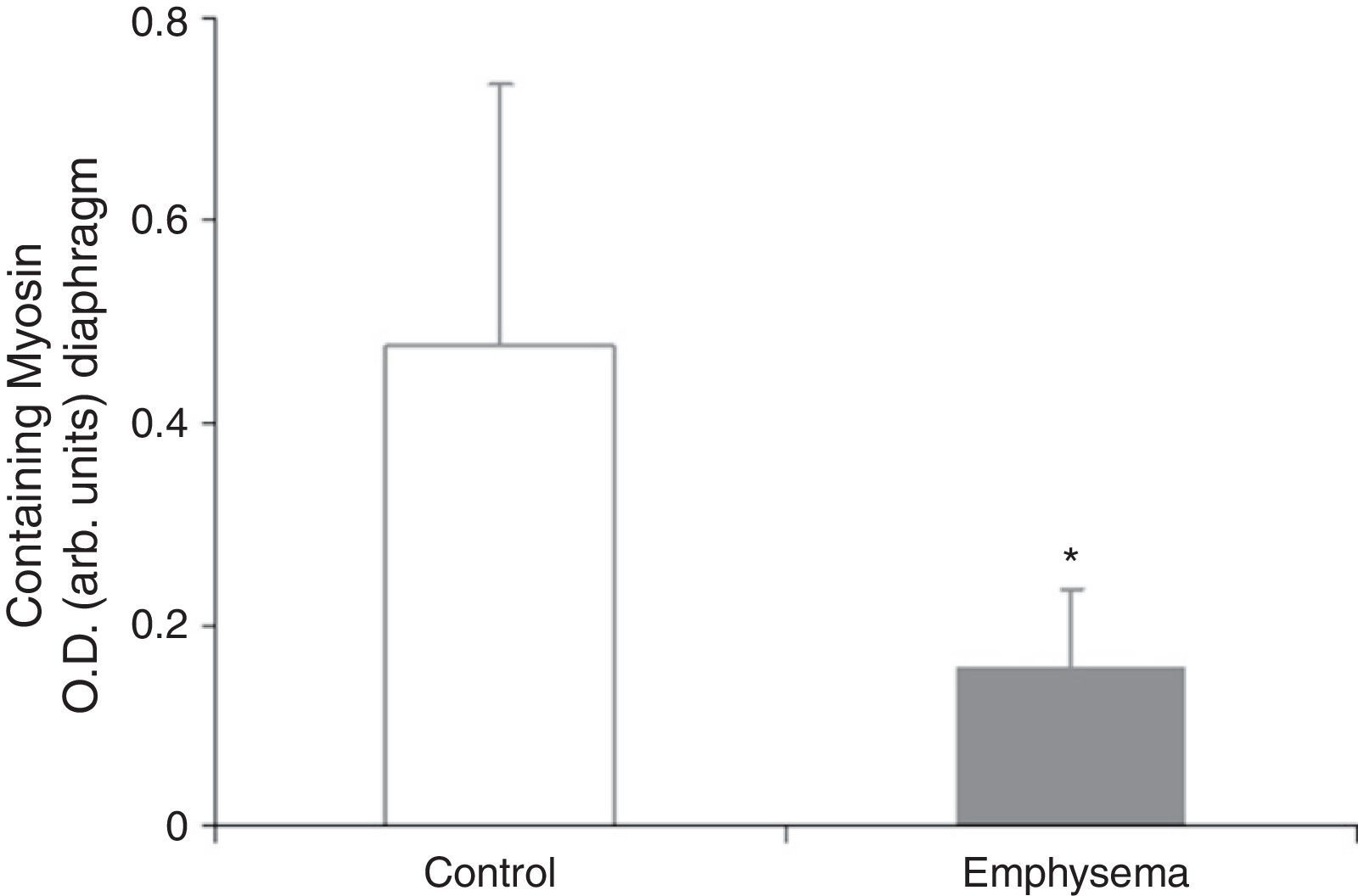
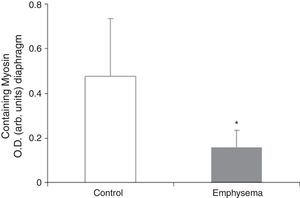
Muscle Proteins Susceptible to DegradationA significant reduction in diaphragm myosin levels (67%) was observed in the mice with emphysema, compared to the controls (Fig. 6), but not in the gastrocnemius (Table 3). Between the 2 groups of animals, no significant differences were shown in either respiratory or peripheral muscle levels of actin and creatine kinase protein (Table 3).
DiscussionThe most notable findings of our research are summarized below. In the diaphragm of mice with emphysema, compared to the control animals, the following was observed: 1) loss of structural protein content, such as myosin; 2) increased protein levels of major cellular signalling pathways, such as myostatin, MAPKs, and transcription factor FoxO1; 3) a drop in myogenin levels; and 4) increased protein oxidation levels, reduced mitochondrial antioxidants, and increased mitochondrial antioxidants in the cytosol. In the peripheral muscle, the most important findings were: 1) an increase in transcription factors such as myostatin that are involved in atrophy; 2) increased protein levels of components of the JNK and NF-kB cellular signalling pathways; and 3) reduced levels of the antioxidant peroxiredoxin-3.
The experimental model used in our study could be thought of as a model of emphysema that, in a way, has features in common with the loss of muscle mass seen in patients with COPD. Thus, over the course of the study, it was observed that the mice with emphysema induced by elastase instillation gained less total body weight than the control animals. Also, the diaphragm and gastrocnemius muscle were of smaller size in the diseased animals than that in the controls. In our study, we thought it would be of interest to identify the molecular mechanisms that resulted in less total body weight gain and less muscle mass development in the mice with emphysema. There is the possibility that these molecular mechanisms correspond to the underlying mechanisms in the muscles of patients with COPD and cachexia. If so, then this animal model could prove to be of great benefit, in the near future, in designing therapeutic objectives for mitigating the cachexia in patients with COPD. Following analysis of the protein levels of different markers that could be involved in the mechanisms causing this cachexia in the mice with emphysema, it can be seen that the diaphragm and the gastrocnemius muscle have different patterns, as described below.
Signalling Pathways and ProteolysisFoxO1 protein—a transcription factor involved in the IGF-1/AKT pathway and regulator of the expression of genes, such as atrogin-1 that are involved in protein degradation pathways and muscle atrophy18,19—was observed to be at increased levels in the diaphragm of emphysematous mice. In a previous study,20 transgenic mice characterized by overexpression of FoxO1 were bred; these, in comparison with control mice, lost weight and were noted to have reduced muscle mass in various muscles of the extremities.20 Diminished expression of genes related to structural proteins of type I fibre was also detected.20 From a histological standpoint, a reduction in the size of type I and II fibres was observed, as well as a decrease in the percentage of type I fibres and an increase in protein catabolism.20 It has been shown that there is increased expression of FoxO in the diaphragm of patients with COPD,12 as well as in the diaphragm of patients exposed to prolonged mechanical ventilation.13 However, peripheral muscle FoxO levels in the mice with emphysema were no different from those in the control animals. It could be concluded that, in experimental murine models and in patients with COPD, the FoxO transcription factor appears to play a major role in signalling increased protein degradation in skeletal muscle.
Four subfamilies of the MAPK pathway have been described: ERK1/2, JNK, ERK5, and p38. Among various cellular functions, this signalling pathway is involved in protein degradation.21 Compared to the control animals, ERK1/2 levels were significantly higher in the diaphragm of mice with emphysema, while JNK levels were higher in their gastrocnemius. In experimental models, both JNK and ERK1/2 have been involved in the signalling of cellular proteolytic systems.21 However, in recent research by our group (observations not published), the MAPK signalling pathway was shown to be unchanged in the quadriceps of patients with advanced COPD.
Increased levels of NF-kB subunit p50 protein were also observed in peripheral muscle, but this was not the case in the diaphragm. The NF-kB/IKKB pathway has been studied extensively; among the numerous other functions that have been described is its direct participation in processes and diseases involving muscle atrophy and a reduction in muscle mass.22 Thus, the loss of muscle mass seen in the gastrocnemius of the emphysematous mice could also be mediated by this signalling pathway. This data is consistent with other published data from a study in which increased signalling via the NF-kB pathway was demonstrated in the vastus lateralis of patients with severe COPD and loss of muscle mass.23 Another recent study24 has also shown increased NF-kB expression in the diaphragm of patients with severe COPD. In summary, this signalling pathway appears to play a key role in proteolytic processes in muscle in experimental models and in the respiratory and peripheral muscles of patients with advanced COPD.
In the diaphragm but not in the gastrocnemius of the mice with emphysema, in comparison with the control animals, levels of the muscle growth factor myogenin were reduced while levels of myostatin were increased. These results are clearly consistent with those obtained in a very recent study,24 which reported decreased myogenin levels and increased myostatin levels in the diaphragm of patients with severe and very severe COPD. Myostatin is a potent inhibitor of muscle mass growth, and it is thought that it may inhibit the proliferation and differentiation of muscle precursor cells that could, in turn, have the capacity to positively regulate protein degradation via the ubiquitin–proteasome system.24–26 On the other hand, myogenin is a growth factor that favours the proliferation and differentiation of muscle fibres. Recent data (observations not published) has revealed very similar findings in terms of myostatin and myogenin levels in the vastus lateralis muscle of patients with severe COPD and loss of muscle mass. In fact, it should be kept in mind that our study conforms to a model of less increase in the amount of body weight and muscle mass in the diseased mice rather than a model of weight loss. In this regard, increased levels of the muscle growth inhibitor myostatin in conjunction with reduced levels of the muscle growth potentiating factor myogenin appears to be consistent with our study's experimental model of less weight and muscle gain.
The significant reduction in the respiratory muscle—but not peripheral muscle—content of the contractile protein myosin in the mice with emphysema, compared to the controls, constitutes a major finding of our research. Moreover, in the mice with emphysema, compared to the controls, the percentage for no gain in diaphragm muscle mass was 34%, while for the gastrocnemius it was 21%. These differences between the 2 muscles in the same group of mice could also explain why reduced myogenin and myosin levels were seen only in the respiratory muscles in mice with emphysema, compared to the control animals, and were not seen in the peripheral muscle.
Regarding the lack of differences in content for other structural proteins, besides myosin, that were analysed in our study, the findings were similar to those reported in the group's previous studies of the diaphragm7 and vastus lateralis (observations not published) in patients with severe COPD. In fact, using electron microscopy, it has recently been confirmed that there is a myopathy in the respiratory and peripheral muscles of critically ill patients that affects only the thick filaments of the sarcomeric structure—that is, it affects the myosin filaments but not the actin filaments. This condition is commonly known as thick filament myopathy.27 Along the same line of reasoning, it is suggested that the reduced myosin content detected in the diaphragm of mice with emphysema in our model is analogous to the myopathy affecting only thick filaments of the sarcomere in critically ill patients. Even though, in our work, there was no access to the study of muscle structure due to the limitations explained below (see “Limitations of the Study”), it is conceivable that, if structural changes occurred in the diaphragm of the mice with emphysema, it is very likely that these changes would also affect the myosin filaments.
We must also comment that, in our study, no differences were seen between the 2 groups of mice in terms of the proteolytic pathways analysed (ubiquitinated proteins). The fact that differences were observed in the signalling pathways but not in the markers of proteolysis analysed in our study suggests that other mechanisms of protein degradation, such as calcium-activated proteases, lysosomes, and autophagy, could be involved in the muscles of the mice with emphysema. For reasons related to the size of the muscle specimens, these proteolytic systems were not analysed in our research; however, they could be the object of study in future research.
Oxidative StressOur study also shows increased total levels of muscle protein oxidation (carbonylated proteins and MDA–protein adducts) in the diaphragm but not in the gastrocnemius of the diseased mice, compared to the controls. These results are consistent with those obtained in previous studies by our group5,7 that showed increased protein oxidation levels in the diaphragm of patients with COPD,5 these proteins being creatine kinase, carbonic anhydrase III, actin, and myosin.7 Given that carbonylated proteins and MDA–protein adducts are potent markers of oxidative stress, it could be concluded that the diaphragm of emphysematous mice in our study showed a clear increase in protein oxidation levels. That may be a response to the sustained and intense muscle contractions to which this muscle was subjected in the mice with emphysema throughout the entire course of the study. On the other hand, the relatively limited activity of the peripheral muscle, as opposed to the diaphragm—especially in the animals with emphysema—could explain why there were no changes in oxidative stress levels in the extremity muscle in these animals.
The content of the mitochondrial antioxidant Mn–SOD was reduced in the diaphragm of the diseased mice, leading to the conclusion that mitochondrial antioxidant systems were impaired in the respiratory muscle. That could contribute to increased oxidant production in the mitochondrial compartment because impaired antioxidant systems would not have sufficient capacity to neutralize the abundant production of oxygenated radicals in the diaphragm of the mice with emphysema, chronically subjected to elevated respiratory loads. Therefore, this would result in an equilibrium in favour of the oxidants. It is important to highlight that Mn–SOD and catalase levels were unchanged in the diaphragm of patients with severe COPD, compared to healthy controls.5 In the case of cytoplasmic antioxidant systems, such as CuZn–SOD and peroxiredoxin-2, increased protein levels of these were observed in the diaphragm of the diseased mice. This could be due to the attempt of cellular antioxidant systems to neutralize the excess oxidants but without fully achieving it, given that, in the end, protein oxidation levels were increased.
Then again, it is important to point out that the moderately reduced content of some cytosolic antioxidant systems in the gastrocnemius of the diseased animals ultimately had no effect on the redox equilibrium in the peripheral muscles. These findings are contrary to those reported for the quadriceps of patients with COPD, where an increased content of antioxidant enzymes Mn–SOD and CuZn–SOD has been shown.3,8 Differences between the murine model and patients with COPD in terms of cellular metabolism and muscle activity could explain the variations in the content of various antioxidant enzymes as well as in the redox equilibrium observed, in the end, in each muscle.
Limitations of the StudyThe following are the most notable limitations of our study: First, owing to the lack of histological preparations of the 2 muscles—because of the size of mouse muscle specimens—it was impossible to analyse potential structural changes, such as the fibrillar composition of each muscle, as well as their sizes. Second, it was also impossible to evaluate muscle function in the animals—again, for experiment logistics reasons related to the animals’ size. In our study, given the specimen size limitations, absolute priority was given, first of all, to an in-depth determination of the molecular mechanisms that may be involved in the loss of muscle mass in this experimental model of pulmonary emphysema. Once the principal molecular mechanisms have been identified and the existence of molecular changes has been confirmed in the muscles of mice with an experimental emphysema, it will be possible, in future studies, to earmark a large portion of the specimens for describing structural changes that may be present in the respiratory and peripheral muscles in mice with emphysema.
ConclusionsThe reduced myosin content observed in the diaphragm of mice with emphysema could explain why it is smaller in these animals. Oxidative stress, myostatin, and other cellular signalling pathways, such as FoxO, appear to be involved in the loss of this structural protein in the respiratory muscle. Differences in metabolism and activity between the respiratory muscle—subjected to sustained contractions throughout the entire study—and the extremity muscle—with relatively limited activity—could explain the variations found in terms of myosin content, oxidative stress levels, and signalling pathway levels, as well as the extent to which muscle mass was lost in each type of muscle.
FinancingThis study was subsidized by SEPAR 2009, MTV3-07-1010, 2009-SGR-393, FIS 06/1043, FIS 07/0751 (FIS), CIBERES, and RTICC RD06/0020/0066 (ISCIII, Ministerio de Ciencia e Innovación), and UTE-Project CIMA.
Conflicts of InterestThe authors have no conflicts of interest to declare.
The authors wish to express their utmost gratitude for the invaluable collaboration of researchers Prof. Luis M. Montuenga, Dr. David Blanco, and Gabriel de Biurrun, of the Laboratorio de Biomarcadores (Área de Oncología, Centro de Investigación Médica Aplicada, Universidad de Navarra, Pamplona, Navarra), for developing the experimental model, for releasing the muscle specimens, and for a critical reading of the manuscript.
Please cite this article as: Fermoselle C, et al. Reducción de la masa muscular mediada por miostatina en un modelo experimental de enfisema pulmonar. Arch Bronconeumol. 2011;47:590–8.