Paragonimiasis is a food-borne zoonosis caused by a trematode of the genus Paragonimus.1,2 Infestation is rare in Spain, but the influx of people from endemic areas should make us keep this condition in the differential diagnosis of our patients.2,5
We report the case of a patient from Ecuador and resident in Spain for 7years with active pulmonary tuberculosis on arrival in Spain and later diagnosed with pulmonary paragonimiasis due to persistent haemoptysis. The diagnosis was established by surgical lung specimen showing granulomas containing parasite eggs and the macroscopic view of the fluke within a lung cavity. Initial tuberculosis treatment and current treatment with praziquantel controlled both conditions.
La paragonimiasis es una zoonosis de transmisión alimentaria causada por un trematodo del género Paragonimus.1,2 Se trata de infestación excepcional en España, pero la afluencia de personas originarias de áreas endémicas debe hacernos tener presente esta entidad en el diagnóstico diferencial de nuestros pacientes.2,5
Presentamos el caso de un paciente natural de Ecuador y residente en España desde hace 7 años con tuberculosis pulmonar activa a su llegada a España y posterior diagnóstico de paragonimiasis pulmonar a raíz de hemoptisis persistente. El diagnóstico se estableció por muestra quirúrgica pulmonar, objetivando granulomas, englobando los huevos del parásito, así como la visualización macroscópica del trematodo dentro de una cavidad. El tratamiento antituberculoso inicialmente y con prazicuantel en la actualidad controló ambas entidades.
Paragonimiasis (also known as pulmonary distomatosis or lung fluke) is a parasitic disease caused by a trematode of the genus Paragonimus whose geographic distribution is limited to Asia (predominant location), West-Central Africa, and Central and South America.1–3,5 Estimates are that it affects some 22 million people around the world,1,2 the most common species being Paragonimus westermani.1,2 In Asia, this is the most prevalent species, while in Africa Poroderma africanum and Paragonimus uterobilateralis are the most common.1,2
Like all trematode infections, paragonimiasis is a food-borne zoonosis,1,2 with a parasitic cycle requiring 2 intermediate hosts—first, a mollusc (river snail), where the embryonated eggs become cercariae, and then a freshwater crustacean (crayfish), where they evolve to metacercariae, which are passed to the definitive host (human being or carnivorous mammal) when these crustaceans are ingested in an undercooked state. They excyst in the digestive tract and mechanically penetrate the duodenum; having reached the peritoneum (peritoneal incubation phase 2–20 days following ingestion, with mild, non-specific symptoms of peritonitis), they will migrate from there to the different organs.1,2 They may migrate to skin, muscle, central nervous system, liver, spleen, and pericardium, among other sites,3,4 lungs and pleura being the most commonly affected.3,4 In the initial phase of the parasitosis (8–10 weeks), they penetrate the diaphragm, with resulting pleural involvement in the form of an eosinophilic exudate, pneumothorax, pleuritic pain, etc., and then invade the lungs, causing occasional cough, low-grade fever, blood-tinged sputum, and migratory infiltrates, along with a 10%–30% peripheral eosinophilia.3,5 After this, a late phase of the parasitosis begins, which may last from 6 to 20 years, with encystment in the parenchyma and formation of small peripheral cavities and tunnels through which they move that, on X-ray, resemble bronchiectases or fibrous tracts. It is in this phase that they begin to produce large numbers of eggs (some 20,000 per day). The clinical features of this phase may include a persistent or recurring rusty-chocolate-brown expectoration with no other symptomatology, along with a persistent minimal eosinophilia or no eosinophilia.
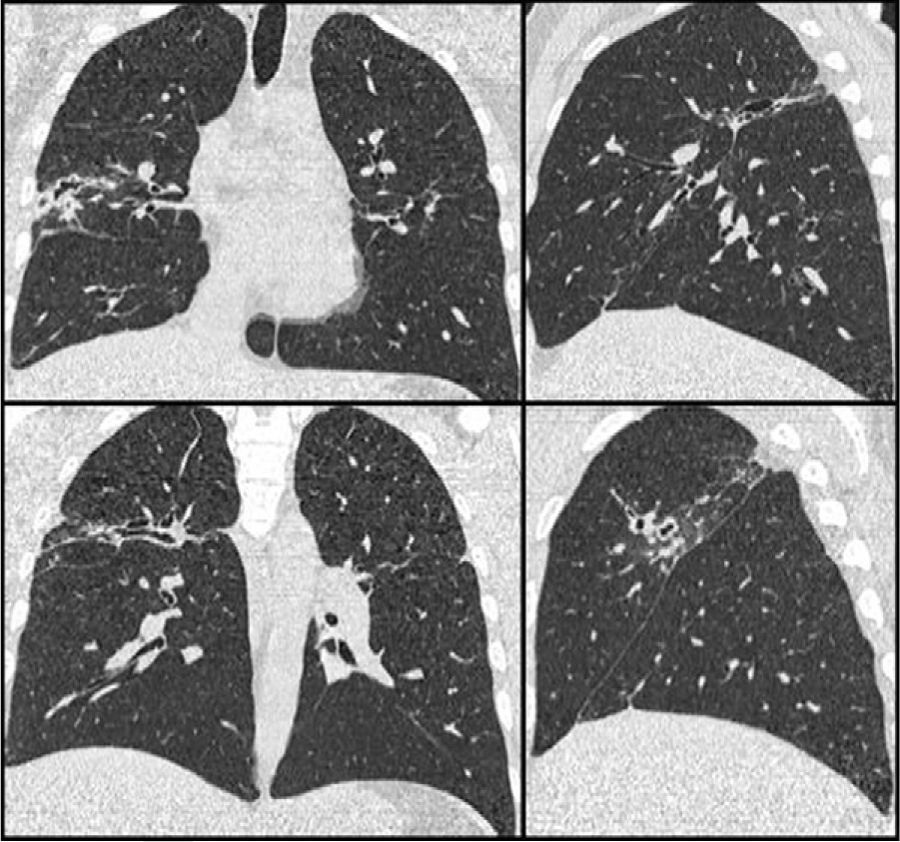
From a radiographic standpoint, this late phase may be entirely normal (20%) or it may present with 1 or several annular cystic peripheral lesions of variable size and wall thickness; these may be accompanied by linear peripheral tracts, which are the tunnels for movement.3,5 Localized masses or nodules may also be found, as well as thickening of the pleura adjacent to these lesions.3,5
We will now present the case of a patient with pulmonary paragonimiasis who previously had pulmonary tuberculosis; these commonly associated conditions complicated the diagnosis of parasitic disease, in this case, delaying it for several years.
Clinical CaseThe patient was a 33-year-old male, with features of Andean ethnicity, from Guayaquil, Ecuador, who had lived in Spain since 2003. That year, on the basis of constitutional symptoms, haemoptysis, and infiltrates with cavitation in the upper lobes, tuberculous disease was confirmed at another Centre by sputum positive for Mycobacterium tuberculosis. He was admitted to a specialized Centre for 6 months of treatment: isoniazid, rifampicin, pyrazinamide, and ethambutol for 2 months, and then isoniazid and rifampicin for 4 more months. After this, the patient had periodic check-ups at another Centre, where he was reported to be asymptomatic since 2003, with the exception of rusty sputum. In 2007, in the course of this follow-up, he had a CAT scan that showed a medium-walled, 2-cm peripheral cavity with images suggestive of cylindrical bronchiectases in the posterior segment of the right upper lobe. Laboratory tests on that date showed a moderate eosinophilia of 9%. Subsequently, in 2008, when he came to our clinic for persistent rusty expectoration (which the patient described as “blood” amounting to about 5-10mL/day), a chest X-ray was taken, the findings of which were superimposable on those of the 2007 CAT scan (Fig. 1). Serial sputums were negative for acid-alcohol resistant bacilli and Löwenstein negative; a Brevundimonas vesicularis was isolated from one of them, for which the patient was started on eradicating treatment with ciprofloxacin 750mg every 12h for 21 days, but the rusty expectoration did not subside. In view of the persistence of symptoms, another chest CAT scan was done, with findings identical to those of the 2007 CAT scan (Fig. 1), and in view of the persistent mild haemoptysis presumably due to bronchiectases and localized cicatricial tracts, and the decision was made to perform a right upper lobectomy, based on normal respiratory function studies.
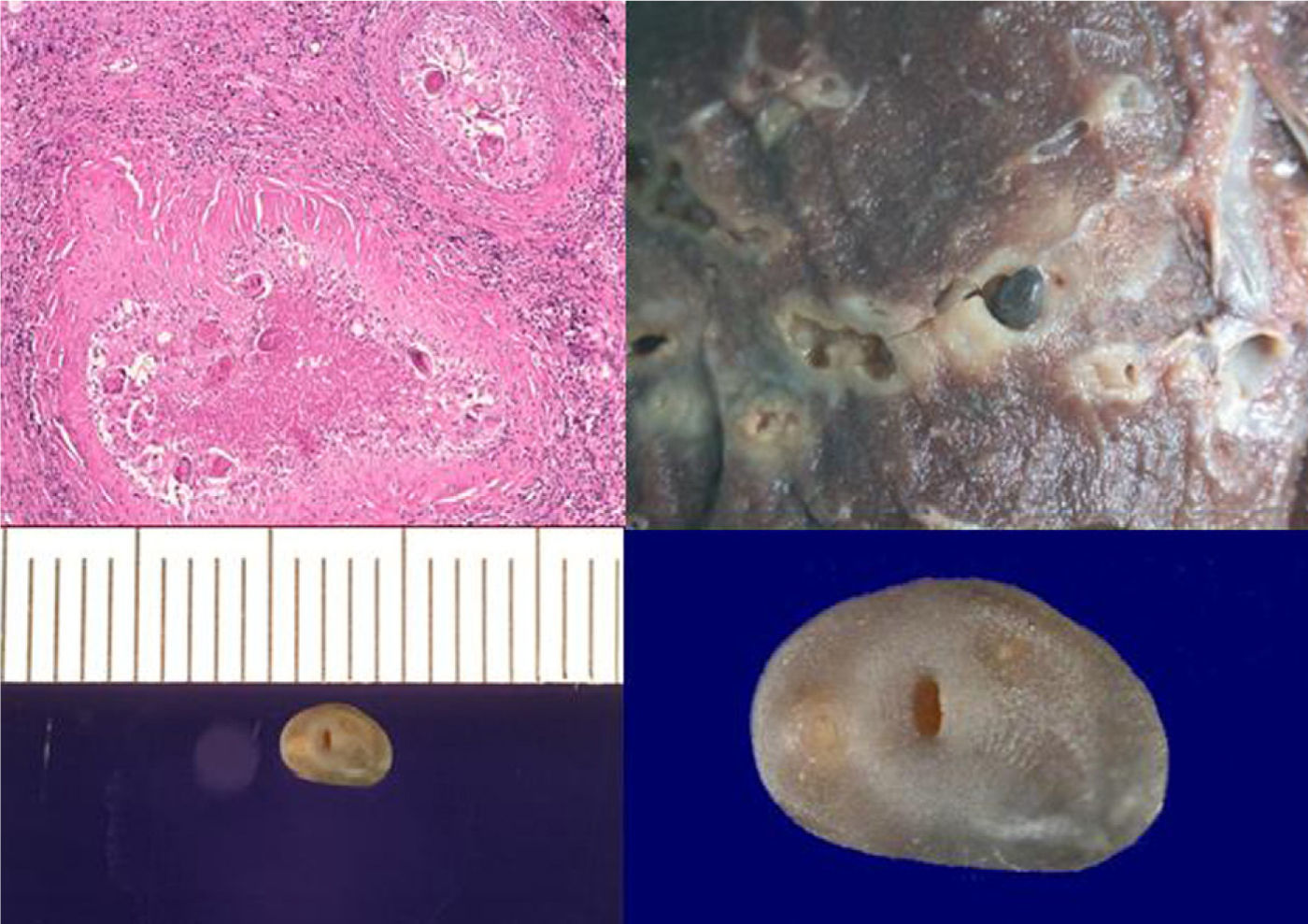
Microscopically, the specimen showed abundant histiocytic cell granulomas with significant peripheral fibrosis and fine lymphocytic crown enclosing extensive necrosis and cavitation, with numerous, yellowish-brown ovoid bodies that were operculate and birefringent with polarized light—characteristic of P. uterobilateralis eggs (Fig. 2). Upon macroscopic examination of the specimen, cavities and tunnels were observed, with significant necrosis and peripheral fibrosis, and an adult P. uterobilateralis specimen was found in one of them (Fig. 2).
Being questioned again about dietary habits in his country, the patient stated that they catch and eat crayfish occasionally. The patient was treated with praziquantel 25mg/kg/8h for 48h6 with good tolerance, no expectoration following the surgery, and no eggs in follow-up sputums.
DiscussionParagonimiasis is a condition documented anecdotally in our country—there are only 2 cases found, described in 19922,5 and in 2008.10 These 3 cases—the 1 we are reporting and the 2 found in the literature—are cases imported from high-risk areas; no cases endemic to our country have been described. Bearing in mind, however, that this is a very common pathology around the world, with an estimated 22 million cases,1,2 it is a condition that should come to mind in non-endemic regions experiencing a high rate of immigration from areas where it is prevalent.
The finding of an association between paragonimiasis and tuberculosis has often been reported,5,6 but there is not enough information to be able to explain the reason for this association. Our interpretation would be that, while the incidence of tuberculosis in these patients is probably similar to that seen in the general population in their country of origin, the fact that the radiographic lesions of these 2 nosological entities are similar means that practically all patients infected with pulmonary paragonimiasis would be routinely screened for tuberculosis, thereby bringing this association to light.
In this particular case, 7 years ago, the radiographic lesions pointed toward the diagnosis of tuberculosis, which was confirmed by sputum culture (bacilloscopy and Löwenstein positive), and the persistent radiographic images of bronchiectases and residual cicatricial tracts were classified as this pathology. After 7 years, given the persistent rusty sputum (which the patient incorrectly perceived and reported as daily haemoptysis), despite the radiographic stability of the lesions, and in view of the life-threatening risk posed by a haemoptysis over time, the decision was made to perform a right upper lobectomy, thereby confirming the diagnosis of paragonimiasis.
The diagnosis, in this case, could have been made by the direct, microscopic visualization of parasite eggs in the sputum,1,3–5 but this pathology, being virtually non-existent in our country, was not suspected, and so it was not looked for—the sputum was not examined under a microscope. Although microscopic visualization of the sputum was done when serial bacilloscopy of the sputum was done, it must be reported that the reagents used in the Ziehl-Neelsen stain destroy Paragonimus spp. eggs. Bronchoscopy and cytology analysis of the bronchial aspirate or bronchial and bronchoalveolar washings would have allowed the eggs to be detected.3–5
Oral praziquantel therapy (25mg/kg/8h for 48h) has a nearly 100% curing rate7 and is well tolerated (occasional headache and somnolence).7 Triclabendazole may be used as an alternative (10mg/kg, single dose); it has an effectiveness of 80%–100%8,9 and is better tolerated than praziquantel.8,9
In conclusion, the diagnosis of different parasitic diseases of the lungs should come to our minds with patients who are natives of endemic regions and have similar lung lesions and eosinophilia, even when mycobacteria are isolated in respiratory specimens. Moreover, in view of all these premises, it may be advisable to perform sputum cytology on a routine basis as screening for parasitic lung disease such as paragonimiasis.
Conflict of InterestsThe authors declare having no conflict of interests.
Please cite this article as: Gómez-Seco J, et al. Paragonimiasis pulmonar. Arch Bronconeumol. 2011;47:610--2.