Clinical probability scores (CPS) determine the pre-test probability of pulmonary embolism (PE) and assess the need for the tests required in these patients. Our objective is to investigate if PE is diagnosed according to clinical practice guidelines.
Materials and methodsRetrospective study of clinically suspected PE in the emergency department between January 2010 and December 2012. A D-dimer value ≥500ng/ml was considered positive. PE was diagnosed on the basis of the multislice computed tomography angiography and, to a lesser extent, with other imaging techniques. The CPS used was the revised Geneva scoring system.
ResultsThere were 3924 cases of suspected PE (56% female). Diagnosis was determined in 360 patients (9.2%) and the incidence was 30.6 cases per 100000 inhabitants/year. Sensitivity and the negative predictive value of the D-dimer test were 98.7% and 99.2% respectively. CPS was calculated in only 24 cases (0.6%) and diagnostic algorithms were not followed in 2125 patients (54.2%): in 682 (17.4%) because clinical probability could not be estimated and in 482 (37.6%), 852 (46.4%) and 109 (87.9%) with low, intermediate and high clinical probability, respectively, because the diagnostic algorithms for these probabilities were not applied.
ConclusionsCPS are rarely calculated in the diagnosis of PE and the diagnostic algorithm is rarely used in clinical practice. This may result in procedures with potential significant side effects being unnecessarily performed or a high risk of underdiagnosis.
Las escalas de probabilidad clínica (EPC) determinan la probabilidad pretest de embolia pulmonar (EP) y valoran la necesidad de las pruebas a realizar en estos pacientes. Nuestro objetivo es investigar si el diagnóstico de EP se realiza de acuerdo a las guías de práctica clínica.
Material y métodosEstudio retrospectivo de las sospechas clínicas de EP en el servicio de urgencias entre enero de 2010 y diciembre de 2012. Se consideró positivo un dímero-D ≥ 500 ng/ml. El diagnóstico de EP se hizo en función de la angiotomografía computarizada multicorte y, en menor medida, por otras técnicas de imagen. La EPC utilizada fue la de Ginebra revisada.
ResultadosLas sospechas de EP fueron 3.924 (56% mujeres). El diagnóstico se estableció en 360 pacientes (9,2%) y la incidencia fue de 30,6 casos/100.000 habitantes/año. La sensibilidad y valor predictivo negativo del dímero-D fueron 98,7 y 99,2% respectivamente. La EPC solamente se calculó en 24 casos (0,6%) y los algoritmos diagnósticos no se siguieron en 2.125 pacientes (54,2%): en 682 (17,4%) porque no se pudo estimar la probabilidad clínica y en 482 (37,6%), 852 (46,4%) y 109 (87,9%) con probabilidad clínica baja, intermedia y alta respectivamente, porque no se aplicaron los algoritmos diagnósticos para tales probabilidades.
ConclusionesLas EPC para el diagnóstico de la EP raramente se calculan y el seguimiento del algoritmo diagnóstico en la práctica clínica es bajo. Esto puede ocasionar el realizar técnicas innecesarias que pueden dar lugar a importantes efectos secundarios, o a incurrir en un elevado riesgo de infradiagnóstico.
Clinical probability scores (CPS) are reliable, noninvasive tools that, based on history and clinical findings, determine pretest probability and assess the need to perform various diagnostic tests in patients with suspected pulmonary embolism (PE).
Different CPS models, including the Geneva revised score, have been validated for PE diagnosis.1–4 These scores, used as part of a diagnostic algorithm in combination with the determination of D-dimer (DD) levels, may help exclude PE in low risk groups and make further tests to rule out this diagnosis unnecessary.5–12 Although it is well accepted that imaging tests should only be carried out when there is a high clinical probability (CP) of PE,10,12 guidelines are clearly not followed, and multislice computed tomography angiography (MSCT) of lung or ventilation-perfusion scintigraphy (V/Q scan) is performed as a first step in PE diagnosis.9,11 Thus, several studies in recent years have shown positivity rates in MSCT of less than 10% in patients with suspected PE,13–16 while prospective clinical trials conducted two decades ago, in which pretest clinical evaluations were carried out before V/Q scan, revealed the disease in at least one third of the patients.17,18 These results suggest overuse of the technique, and possibly poor selection criteria. The probability of confirming positive PE on MSCT in patients without risk factors is extremely small (0.95%). Therefore, it seems that MSCT is probably unnecessary in this scenario,19 and the indiscriminate use of MSCT raises concerns regarding increased exposure to radiation.9
Our hypothesis is that CPS are poorly implemented in practice, and diagnostic protocols are not being applied. The objectives of this study were to determine the degree of compliance with CPS and diagnostic algorithms in clinical practice in our hospital in cases of suspected PE.
Materials and MethodsPatient SelectionThe clinical records of patients attending the emergency department (ED) of a tertiary hospital, serving a population of 392359 inhabitants, for suspected PE were retrospectively reviewed. The study period was from January 2010 to December 2012. The search was made, focusing on DD and MSCT requests from the ED. Tests requested for suspected deep vein thrombosis or causes other than suspected PE were excluded.
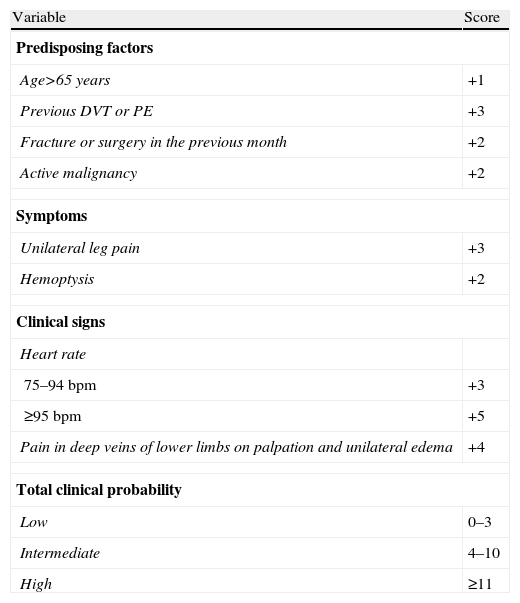
We evaluated whether the revised Geneva CPS had been applied to these patients during the diagnostic process, or whether the necessary data were available in the medical record for a posteriori calculation (Table 1).4 Once the CPS was calculated, it was determined whether the adequate diagnostic algorithm had been followed.20
Revised Geneva Score.
| Variable | Score |
| Predisposing factors | |
| Age>65 years | +1 |
| Previous DVT or PE | +3 |
| Fracture or surgery in the previous month | +2 |
| Active malignancy | +2 |
| Symptoms | |
| Unilateral leg pain | +3 |
| Hemoptysis | +2 |
| Clinical signs | |
| Heart rate | |
| 75–94bpm | +3 |
| ≥95bpm | +5 |
| Pain in deep veins of lower limbs on palpation and unilateral edema | +4 |
| Total clinical probability | |
| Low | 0–3 |
| Intermediate | 4–10 |
| High | ≥11 |
PE, pulmonary embolism; DVT, deep venous thrombosis; bpm, beats per minute.
PE diagnosis was established by high probability results in a V/Q scan (according to PIOPED study criteria: two or more large segmental perfusion defects [>75% of a segment] without abnormalities in the V/Q scan or chest X-ray or defects substantially greater than the ventilation defects or concurrent radiological abnormalities; two or more moderate segmental perfusion defects [>25% and ≤75% of a segment] without concurrent scan abnormalities in the V/Q scan or chest X-ray, with a large non-concurrent segmental defect perfusion; four or more moderate segmental perfusion defects without changes in the V/Q scan or chest X-ray)17; by compression ultrasonography of the lower limbs, showing proximal deep venous thrombosis in patients with non-diagnostic findings in V/Q scan21; or diagnostic chest MSCT.22 V/Q scan was performed only if there was a risk of contrast nephropathy when performing MSCT23 (serum creatinine >1.3mg/dl, normal range: 0.4–1.1mg/dl).
D-dimer AnalysisDD in serum was determined using D-Dimer HemosIL HS 500 (Instrumentation Laboratory, Milan, Italy), an immunoassay based on latex particles automated in the ACL TOP 700 (Instrumentation Laboratory, Milan, Italy) (turbidimetric immunoassay). Cutoff for DD was 500ng/ml. The sensitivity and negative predictive value of this test for all CP subsets is 100%, and the lower limit of 95% CI in patients with low to moderate CP is greater than 95%.24
In patients not diagnosed with PE, any alternative diagnosis at ED discharge was recorded. All patients were followed-up for 3 months through their medical history to rule out a recurrent PE.
All patients signed an informed consent prior to pulmonary MSCT or V/Q scan. The study was approved by the Ethics Committee for Clinical Research of Galicia (registry 2012/430).
Statistical AnalysisDescriptive analysis of the variables included calculation of percentages for qualitative variables, and mean, median, standard deviation and range for quantitative variables. Qualitative variables were compared using the Chi-square (χ2) test. Data analysis was carried out with the statistics package SPSS version 18.0 for Windows. Significance level was set at 0.05 for all analyses.
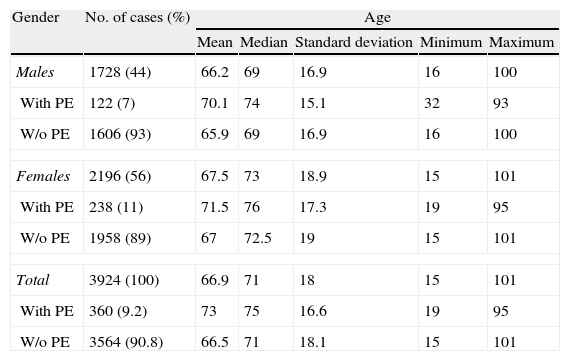
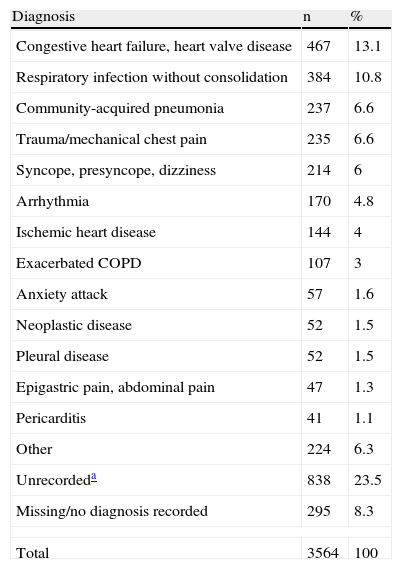
ResultsDuring the study period, 3924 cases of suspected PE were identified (56% female), and the diagnosis was confirmed in 360 patients (9.2%). Demographic data are shown in Table 2. Table 3 contains the most common diagnoses of patients without PE.
Demographic Data of Patients Studied.
| Gender | No. of cases (%) | Age | ||||
| Mean | Median | Standard deviation | Minimum | Maximum | ||
| Males | 1728 (44) | 66.2 | 69 | 16.9 | 16 | 100 |
| With PE | 122 (7) | 70.1 | 74 | 15.1 | 32 | 93 |
| W/o PE | 1606 (93) | 65.9 | 69 | 16.9 | 16 | 100 |
| Females | 2196 (56) | 67.5 | 73 | 18.9 | 15 | 101 |
| With PE | 238 (11) | 71.5 | 76 | 17.3 | 19 | 95 |
| W/o PE | 1958 (89) | 67 | 72.5 | 19 | 15 | 101 |
| Total | 3924 (100) | 66.9 | 71 | 18 | 15 | 101 |
| With PE | 360 (9.2) | 73 | 75 | 16.6 | 19 | 95 |
| W/o PE | 3564 (90.8) | 66.5 | 71 | 18.1 | 15 | 101 |
PE, pulmonary embolism.
Alternative Diagnoses to Pulmonary Embolism.
| Diagnosis | n | % |
| Congestive heart failure, heart valve disease | 467 | 13.1 |
| Respiratory infection without consolidation | 384 | 10.8 |
| Community-acquired pneumonia | 237 | 6.6 |
| Trauma/mechanical chest pain | 235 | 6.6 |
| Syncope, presyncope, dizziness | 214 | 6 |
| Arrhythmia | 170 | 4.8 |
| Ischemic heart disease | 144 | 4 |
| Exacerbated COPD | 107 | 3 |
| Anxiety attack | 57 | 1.6 |
| Neoplastic disease | 52 | 1.5 |
| Pleural disease | 52 | 1.5 |
| Epigastric pain, abdominal pain | 47 | 1.3 |
| Pericarditis | 41 | 1.1 |
| Other | 224 | 6.3 |
| Unrecordeda | 838 | 23.5 |
| Missing/no diagnosis recorded | 295 | 8.3 |
| Total | 3564 | 100 |
COPD, chronic obstructive pulmonary disease.
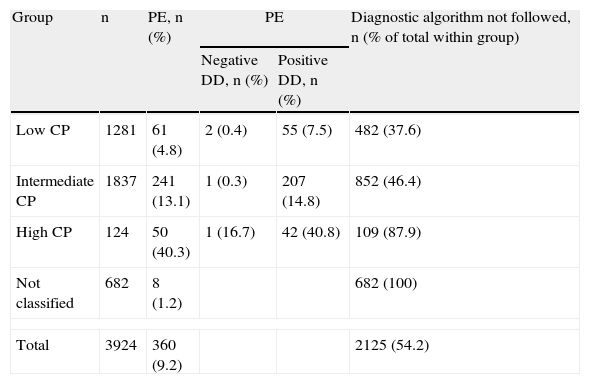
CPS had been recorded in the clinical history of only 24 patients (0.6%). In another 3218 (82%), this could be calculated a posteriori. Finally, data from the clinical history were insufficient for making the calculation in 682 cases (17.4%). Of the 3242 patients in whom CP was determined, 1281 (39.5%) had low probability, 1837 (56.7%) intermediate, and 124 (3.8%) high (Table 4).
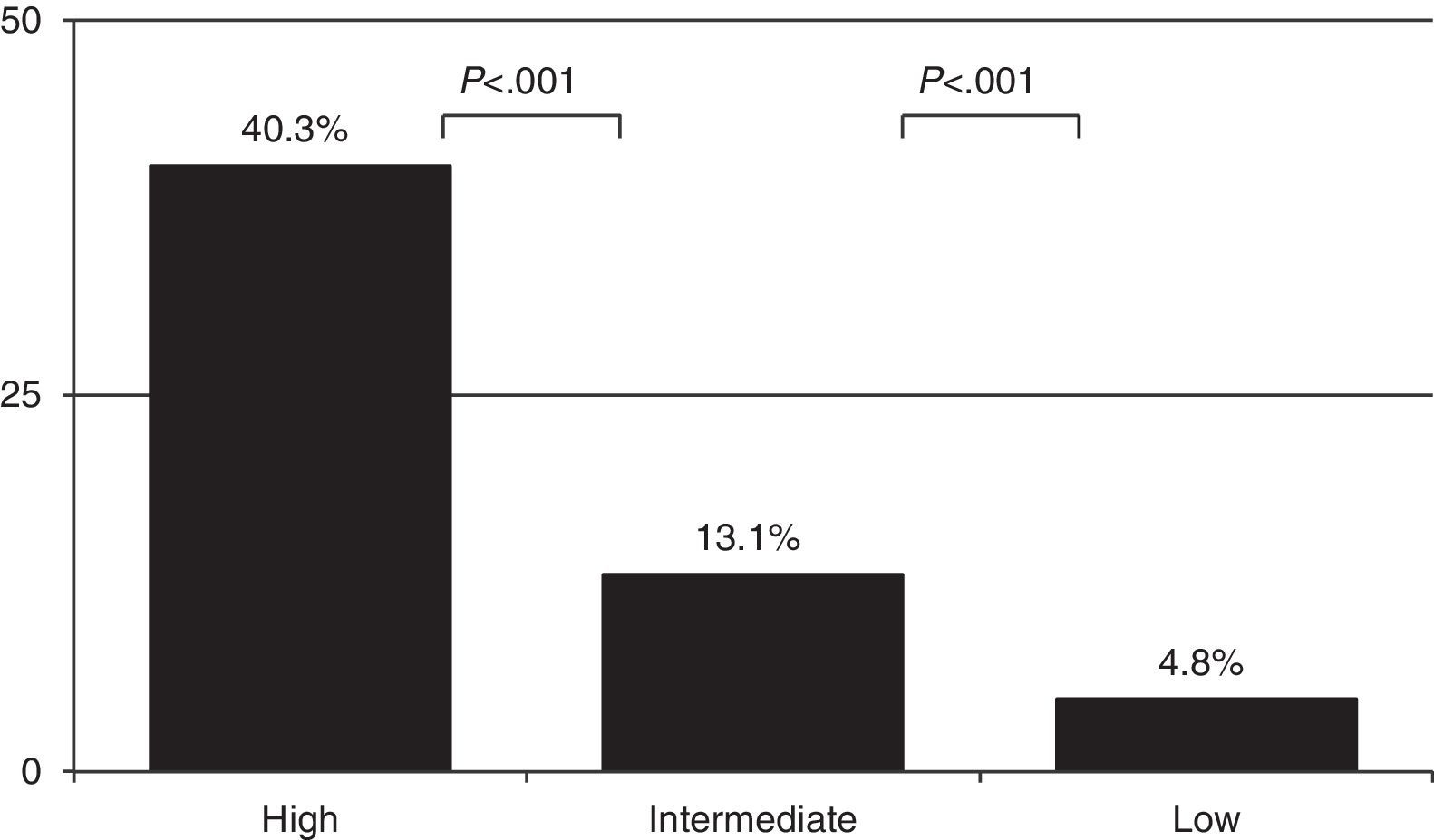
Number of Patients Included by Probability Group; Number of Patients With Pulmonary Embolism (With and Without D-dimer Results) and Patients in Whom the Diagnosis Algorithm was not Followed.
| Group | n | PE, n (%) | PE | Diagnostic algorithm not followed, n (% of total within group) | |
| Negative DD, n (%) | Positive DD, n (%) | ||||
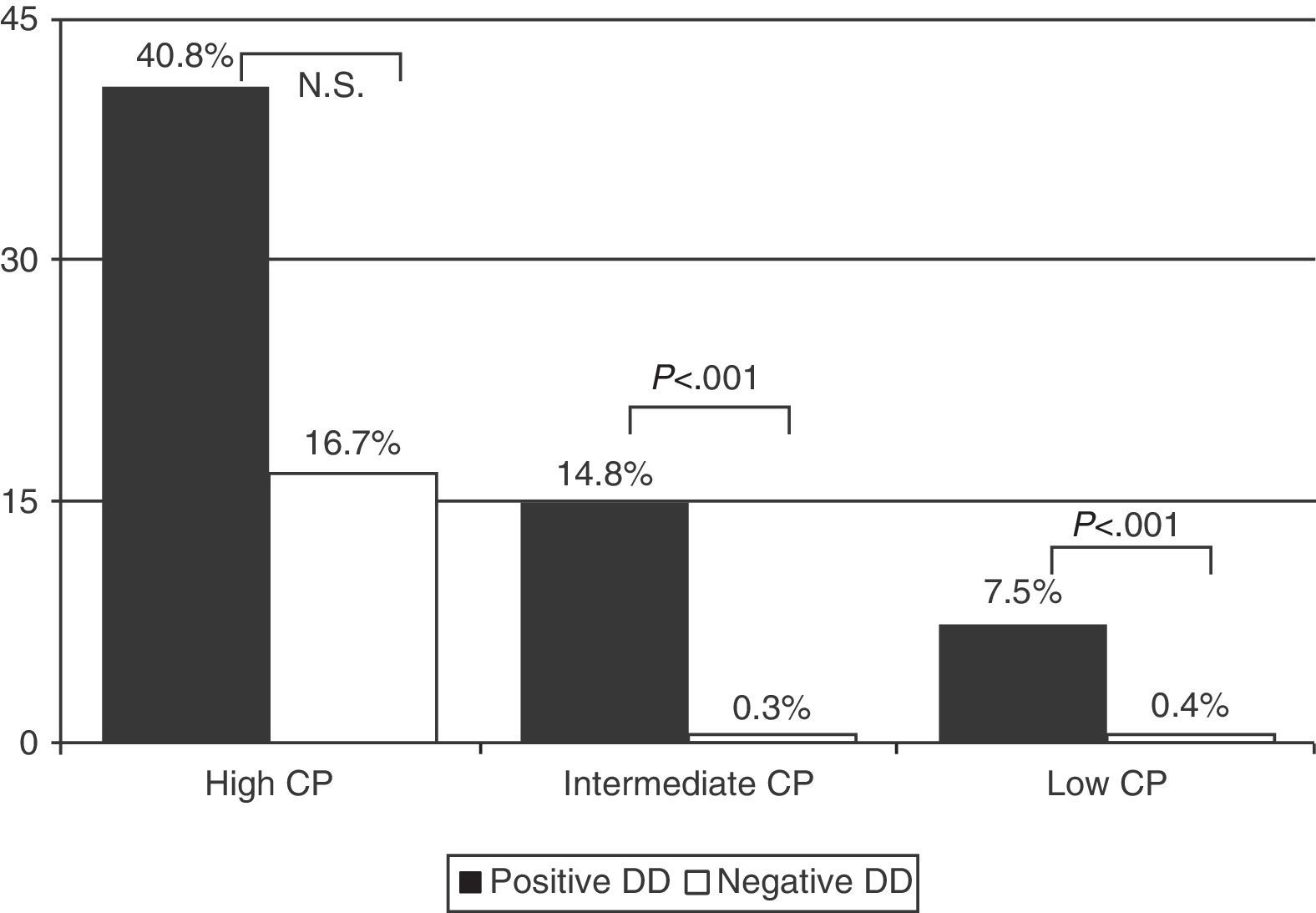
| Low CP | 1281 | 61 (4.8) | 2 (0.4) | 55 (7.5) | 482 (37.6) |
| Intermediate CP | 1837 | 241 (13.1) | 1 (0.3) | 207 (14.8) | 852 (46.4) |
| High CP | 124 | 50 (40.3) | 1 (16.7) | 42 (40.8) | 109 (87.9) |
| Not classified | 682 | 8 (1.2) | 682 (100) | ||
| Total | 3924 | 360 (9.2) | 2125 (54.2) | ||
DD, D-dimer; PE, pulmonary embolism; CP, clinical probability.
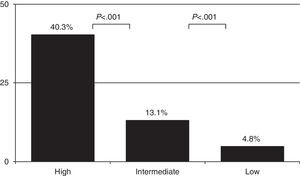
As expected, the prevalence of PE varied depending on CP: 4.8% [61/1281] in the low probability group, 13.1% [241/1837] in the intermediate probability group, and 40.3% (50/124) in the high probability group (Fig. 1) (P<.001 comparing all of them). PE was diagnosed in 8 more cases among the 682 patients in whom CP calculation was not possible (Table 4). Overall prevalence of PE was 9.2% (360/3924) and incidence was 30.6 cases per 100000inhabitants/year.
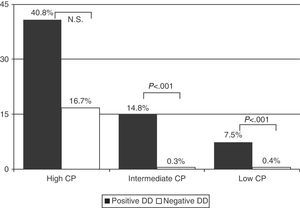
Prevalence of PE in patients with low CP and positive DD was 7.5% (55/737), and 0.4% (2/512) in those with negative DD. In those with intermediate CP, prevalence rates were 14.8% (207/1395) and 0.3% (1/318) and, in those with high CP, the rates were 40.8% (42/103) and 16.7% (1/6), respectively (Fig. 2) (P<.001 for low and intermediate CP, between positive and negative DD, but not significant in high CP) (Table 4).
DD was determined in 94.7% of cases (3071/3242). Its sensitivity for the diagnosis of PE was 98.7%, and the negative predictive value was 99.2%. MSCT was not performed in 70 patients due to the risk of contrast nephropathy.
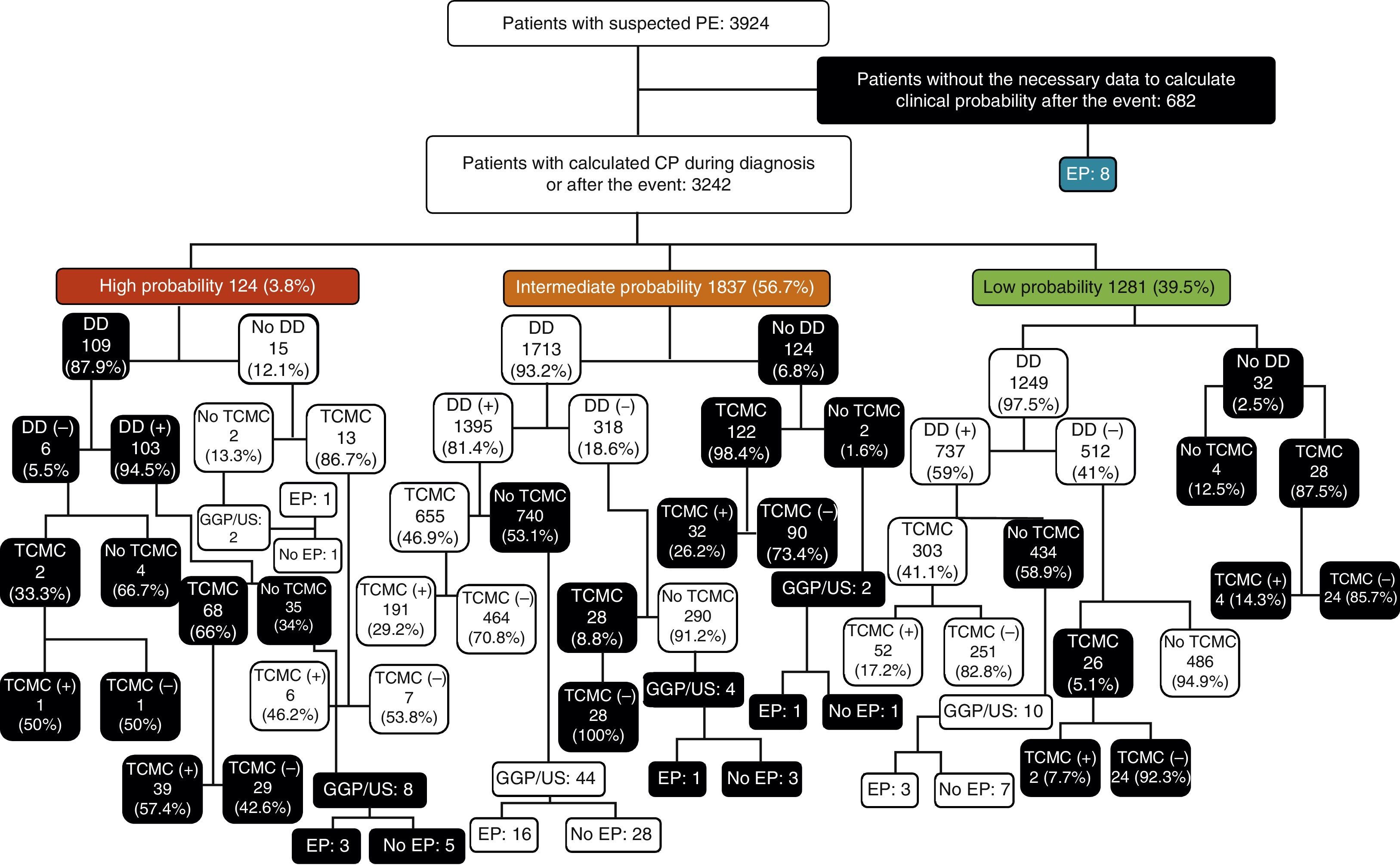
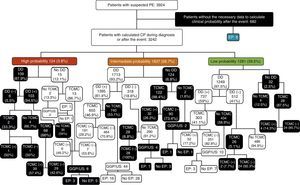
Among patients with low CPS, DD was not determined in 32 cases (2.5%). Of the 1249 in whom it was measured, results were negative in 512 (41%) and positive in 737 (59%). Among those with negative DD, MSCT was performed in 26 cases (5.1%), and this was positive in 2 cases (7.7%). No PE was demonstrated in 486 patients in whom DD was negative and MSCT was not performed. Of those patients who had positive DD (737), MSCT was performed in only 303 (41.1%), of whom 52 (17.2%) had PE. Of the 434 patients who did not have MSCT results, 3 had PE (MSCT was not performed in these 3/10 due to risk of contrast nephropathy). Of the 32 patients in whom DD was not measured, MSCT was performed in 28, of whom 4 had PE, while 4 did not undergo any other diagnostic test and PE was not demonstrated in any of them (Fig. 3).
Patient flowchart according to clinical probability, with the results of diagnostic tests for pulmonary embolism. DD, D-dimer; PE, pulmonary embolism; CPS, clinical probability score; V/Q Scan/US, ventilation-perfusion scintigraphy/perfusion or compression ultrasonography of the lower extremities; MSCT, multislice computed tomography; (−), negative; (+), positive;
Among patients with intermediate CPS, DD was not determined in 124 patients (6.8%). In 1713 in whom DD was measured (93.2%), results were negative in 318 (18.6%) and positive in 1395 (81.4%). In the first group (negative DD), MSCT was performed in 28 cases (8.8%), all of which were negative. Of those in whom MSCT was not done (290), one patient presented PE (1 of the 4 patients in whom MSCT was not performed due to risk of contrast nephropathy). Among those with positive DD, MSCT was performed in 655 (46.9%), and was positive in 191 (29.2%). Of the 740 patients with intermediate CPS and positive DD in whom MSCT was not performed, 16 (2.2%) had PE (16/44 with risk of contrast nephropathy that did not undergo MSCT). Finally, of the 124 patients in whom DD was not obtained, MSCT was performed in 122 (98.4%), of which 32 (26.2%) had PE. Of the 2 patients who did not undergo MSCT due to risk of contrast nephropathy, one had PE (Fig. 3).
Of the 124 patients with high CPS, DD was determined in 109 (87.9%) and found positive in 103 (94.5%). MSCT was performed in only 2 of the 6 patients with negative DD, one of whom had PE. PE was not demonstrated in the 4 patients that did not undergo MSCT. MSCT was performed in 68 (66%) of the 103 patients with positive DD; results were positive in 39 (57.4%). Of the 35 patients in whom MSCT was not performed despite having high CP of PE and positive DD, 3 had PE (3/8 in whom MSCT was not performed due to risk of contrast nephropathy). Of the 15 patients without DD determination, 13 had MSCT, which was positive in 6 (46.2%). Of the 2 patients in whom MSCT and DD were not performed, one presented PE (1/2 in whom MSCT was not performed due to risk of contrast nephropathy) (Fig. 3).
Of the total 3924 patients with suspected PE, CPS and diagnostic algorithms were not followed in 2125 (54.2%): in 682 cases (32.1%) the data required to estimate CP were not collected; DD was not determined in 109 patients (5.1%) with high CP; of 852 cases (40.1%) with intermediate CP, DD was not determined in 124, MSCT was not performed despite positive DD in 696, or MSCT or other diagnostic tests were performed with negative DD (32); and, in the case of the 482 patients (22.7%) with low CP, DD was not determined in 32, MSCT was not performed despite positive DD in 424, or MSCT was performed with negative DD in 26 (Table 4 and Fig. 3).
DiscussionThis study confirms the hypothesis that CPS in the diagnosis of PE is rarely calculated and diagnostic algorithms are often not applied in clinical practice, since these were correctly used in only 45.8% of suspected PE.
Diagnosis of PE requires the use of several diagnostic techniques that should be used sequentially. Since the use of a validated diagnostic study is associated with a substantially decreased risk of complications,25 it is advisable that this diagnosis is carried out in a standardized manner. CPSs are intended to identify patients with intermediate or high CP, because they should receive anticoagulant therapy until the results of diagnostic tests are available.
CP calculation was not possible in 682 patients (17.4%) because the CP history data were insufficient, although 8 patients were diagnosed with PE. The correct diagnostic protocol had apparently not been applied in any of these cases.
DD determination is highly sensitive for ruling out PE if the blood concentration is <500ng/ml, at least in patients with low or intermediate CP.26 MSCT is not indicated in this case, since the probability of PE is low.1,2,6 In this manner, excessive use of MSCT that could cause an increase in cancer incidence attributable to radiation9 and the risk of contrast nephropathy are avoided.23 Therefore, this technique should only be carried out in patients with low or intermediate CP if DD levels are >500ng/ml. For this approach to allow the exclusion of PE with a sufficient safety margin, the method for DD determination should ensure a negative predictive value of >98%.27 In our series, DD sensitivity was 98.7% and its negative predictive value was 99.2% (four false negatives). Among patients with low and intermediate CP in whom DD was <500ng/ml, MSCT was performed in 5.1% (26/512) and 8.8% (28/318) of patients respectively. These results are similar to those obtained by Corwin et al. (7%).28 However, if it is taken into account that MSCT was also directly performed in 28/32 patients with low CP and in 123/124 (99.2%) with intermediate CP with no DD determination, 205 MSCT scans should not have been carried out for one or other reason (20.8% with negative DD or without results), a figure still below the 24% of patients in the series of Dunn et al.29
When CP is low or intermediate and DD >500ng/ml, the diagnostic algorithm states that MSCT or another diagnostic test should be performed to rule out the disease. In our series, this was not done in 52.5% of these patients (1120/2132; 424/737 with low and CP and 696/1395 with intermediate CP). One reason that might explain why the protocol was not followed is that positive DD is nonspecific, and an alternative diagnosis may have been established in some cases (Table 3). However, this would not apply to all cases because, although some of these diagnoses may involve elevated DD,30 most are not related to the cardiopulmonary system. Moreover, a final diagnosis was not established in many others. Corwin et al. found that in 42% of the patients (605/1431) with positive DD in their series, MSCT was not performed.28 This might imply that an unknown percentage of patients with PE were not diagnosed, a possibility that cannot be ruled out. These patients were followed for 3 months through their clinical history, but this was aimed more at confirming that PE did not recur within that time, than at ruling out the possibility that diagnosis was not PE in the first place. PE incidence in our series (30.6 cases per 100000 inhabitants/year) was lower than that reported by other authors.31–33 This could also be attributed to possible underdiagnosis, since MSCT was not performed in these cases.
Among patients with high CP for PE, the diagnostic protocol was applied in a standardized manner in only 15 patients (12.1%), because DD was determined first in 109 (87.9%).
The total number of patients in whom the diagnostic algorithm was not applied was 2125 (54.2%). In a recent study, Weiss et al. found that only 23% of the physicians surveyed used the published prediction scores for assessing pretest probability of PE, and 73% preferred an unstructured approach despite knowing the published guidelines.34 While acknowledging that this pretest probability of PE is empirically evaluated,12 this should not influence the correct application of established diagnostic protocols.
As expected, PE prevalence increased with CPS.35 Similarly, PE prevalence was significantly higher within the same CP (P<.001) when DD was positive, except in the high CP group. Possibly the reason is that only 6 patients had negative DD, compared to 103 with positive DD. Overall PE prevalence was 9.2%, slightly higher than the 6% of Corwin et al.,28 but well below the 27% in the series of Prologo et al.36 This decline in PE prevalence compared to a series of previous decades may be due to the increasingly frequent use of MSCT in emergency services, with a consequent reduction in diagnostic yield. It should be remembered that DD specificity is very low and it is not useful for diagnosing acute PE. If DD is determined in cases where suspected PE is clinically not well supported, elevated results may lead to unnecessary MSCT scans.
The main limitation of our study is its retrospective nature. CPS or compliance with the diagnostic protocol could not be established in 17.4% of cases (682) for this reason. Another limitation is the inability to determine whether any of the patients with a positive DD in whom MSCT was not performed, did actually have PE. Follow-up of the clinical history for 3 months does not exclude the diagnosis at the time that these patients came to the emergency department.
In summary, CPS for PE diagnosis is rarely calculated, and diagnostic protocols are rarely applied in clinical practice in our center. This can lead, in some cases, to the performance of unnecessary imaging techniques that may also lead to serious side effects and, in others, to a high risk of underdiagnosis, since the disease cannot be safely ruled out. If CPS were implemented as part of a diagnostic algorithm, higher diagnostic yield could be achieved, and the available resources could be optimized.
Authors’ ContributionsP. Sanjuán is the writer and author, involved in conception and design, data collection, analysis and interpretation of data and approved the final manuscript.
N. Rodríguez-Núñez is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript
C. Rábade is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
A. Lama is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
L. Ferreiro is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
F.J. González-Barcala is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
J.M. Álvarez-Dobaño is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
M.E. Toubes is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
A. Golpe is the co-author, involved in data collection, critical revision of the manuscript and approved the final manuscript.
L. Valdés is the writer and author, involved in conception and design, analysis and interpretation of data and approved the final manuscript.
FundingThis work has not benefited from any scholarship or financial help.
Conflicts of InterestThe authors declare no conflicts of interest.
Please cite this article as: Sanjuán P, Rodríguez-Núñez N, Rábade C, Lama A, Ferreiro L, González-Barcala FJ, et al. Escalas de probabilidad clínica y algoritmo diagnóstico en la embolia pulmonar: ¿se siguen en la práctica clínica? Arch Bronconeumol. 2014;50:172–178.
Partially presented at the 46th SEPAR National Congress. Barcelona, June 2013.