Immunotherapy (particularly immune checkpoint inhibitors) in the treatment of patients with lung cancer has aroused great interest in recent years, revolutionized the management of patients with locally advanced/metastatic disease, and given hope to both patients and treating physicians. These drugs, in combination or in monotherapy, have become the standard treatment for many patients with lung cancer, and their use is expected to increase significantly in the near future. In this article, we will review the growing importance of imaging techniques in the evaluation of therapeutic response to immunotherapy in lung cancer patients, with emphasis on the new specific radiological criteria on response to immunotherapy, atypical radiological responses (pseudoprogresion, dissociative responses, hyperprogresion), and the main radiological manifestations of adverse events associated with immunotherapy (sarcoid reactions, pulmonary toxicities, etc.). Pulmonologists must be familiar not only with atypical radiological responses to immunotherapy and their prognostic implications, but also with their effects and the new radiological criteria of response to assess treatment response. In this study, we will address key concepts such as “pseudoprogresion”, “paradoxical response”, “hyperprogresion”, or “unconfirmed progression”, and their significance in the management of patients with lung cancer treated with immunotherapy.
En los últimos años la inmunoterapia (particularmente los inhibidores de los puntos de control inmunitario) ha suscitado un gran interés en el tratamiento de los pacientes con cáncer de pulmón, revolucionando el manejo de los pacientes con tumores localmente avanzados/metastásicos y generando esperanzas entre los pacientes y los médicos que diagnostican y tratan a estos enfermos. Estos fármacos se han convertido (combinados o no con otras terapias) en el tratamiento estándar de muchos pacientes con cáncer de pulmón y se espera que su uso aumente significativamente en un futuro próximo. En este manuscrito revisaremos la importancia creciente de las técnicas de imagen en la valoración de respuesta al tratamiento con inmunoterapia de los pacientes con cáncer de pulmón, haciendo hincapié en los nuevos criterios radiológicos específicos de respuesta con inmunoterapia, en las respuestas radiológicas atípicas (seudoprogresión, respuestas disociadas, hiperprogresión) y en las principales manifestaciones radiológicas de los eventos adversos asociados a la inmunoterapia (reacciones sarcoideas, toxicidades pulmonares, etc.). Los neumólogos deben conocer no solo las respuestas radiológicas atípicas de la inmunoterapia y sus implicaciones pronósticas, sino también sus efectos secundarios y los nuevos criterios radiológicos de respuesta desarrollados para valorar la respuesta al tratamiento. En este trabajo se tratarán conceptos claves como «seudoprogresión», «respuesta paradójica», «hiperprogresión» o «progresión no confirmada» y su significado en el manejo de los pacientes con cáncer de pulmón tratados con inmunoterapia.
As a result of the work of recent Nobel laureates James P. Allison and Tasuku Honjo, immunotherapy has become a therapeutic alternative for many cancer patients. This approach was initially explored in melanoma, but the first of the high-incidence tumors in which it has proven effective was non-small cell lung cancer (NSCLC).1
Lung cancer (LC) is a disease characterized by a strongly immunosuppressive environment. Over the years, clinical trials based on that premise have tested different types of vaccines in hundreds of patients, but with little success. Now, however, enormous interest has been generated by the initial results of trials with immune checkpoint inhibitors (ICI). These have been consolidated as more robust data become available, to the point that these compounds now form part of the current therapeutic arsenal in different clinical scenarios of patients with LC.2
This strategy is based on the understanding that our immune system operates complex control mechanisms that allow our bodies to maintain tolerance to our own antigens and avoid inappropriate responses. Tumor cells use these mechanisms to escape the immune system, and until now this activity has been a major constraint in attempts to use immunotherapy strategies. The discovery of checkpoints and ICIs, namely anti-CTLA4 (cytotoxic T lymphocyte-associated antigen-4), anti-PD-1 (programmed cell death protein 1) and anti-PD-l1 (programmed death ligand 1), that act by releasing that brake and facilitating the activation of lymphocytes, marked the beginning of the therapeutic revolution known as oncological immunotherapy.3
Three drugs, nivolumab, pembrolizumab, and atezolizumab, are currently approved in the second-line treatment of patients with advanced LC, and have shown benefit in randomized CTs with respect to objective response rate, overall survival, and a better toxicity profile against the comparator (docetaxel). The CheckMate-017 and CheckMate-057 studies used similar designs to test nivolumab in squamous and non-squamous LC4; the Keynote-010 study used pembrolizumab (without histologic distinction) but only in PD-L1-positive tumors5; and the OAK study used atezolizumab in both histologies without preselecting patients based on tumor PD-L1 expression.6 These CTs showed a 20% response rate in the unselected population and achieved 3- and even 5-year survival rates of 17% and 16%, respectively, results unheard of with conventional chemotherapy treatments.7,8 Recent CTs with both single and combination therapy in different disease stages of LC have provided therapeutic alternatives and led to changes in standard first-line and post-chemotherapy/radiation therapy in patients with locally advanced LC. Pembrolizumab, for example, has been approved in the first-line treatment of 30% of patients whose tumors have >50% PD-L1 expression, and in combination with different chemotherapy regimens based on histology.9–11 Atezolizumab has also been explored in phase III studies with different combinations of chemotherapy, all of which met their primary objective in patients with advanced LC12–14 and in patients with disseminated small cell LC.15 Finally, durvalumab has shown substantial benefit not only in progression-free survival, but also in overall survival after standard treatment (with chemotherapy and radiation therapy) in stage 3 LC.16
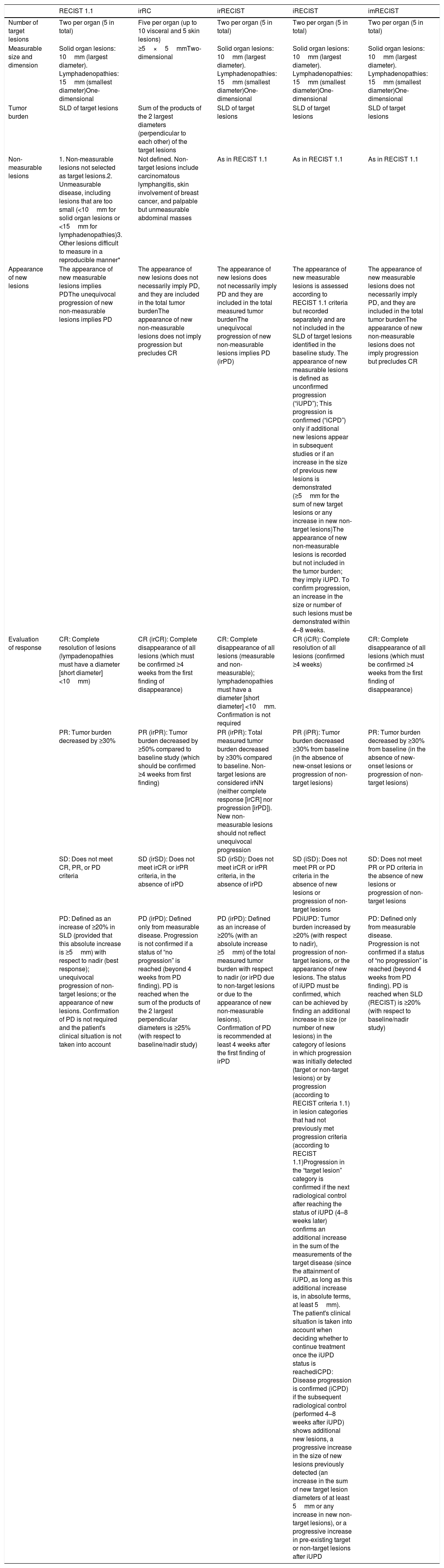
Response CriteriaIn recent decades, several radiological criteria of response to different cancer treatments have been published.17 These criteria must be objective, reproducible, and consistent to provide a common language and accurate assessment of the effects of new therapies.18 Initially, criteria such as solid tumor response assessment (RECIST) or World Health Organization (WHO) were based on cytotoxic chemotherapy treatments, and assumed that an increase in tumor size invariably involved disease progression.19,20 The emergence of immunotherapy has revealed that the standard criteria (RECIST, WHO) may fail to detect atypical response patterns (such as pseudoprogression or paradoxical response).21 In this section, we will review the radiological response criteria with immunotherapy described in recent years that share common aspects, such as the assessment of target and non-target lesions in baseline studies. Overall response is evaluated by quantifying changes in target lesion size, estimating changes in non-target lesions, and documenting the appearance of new lesions, categorized into 4 groups (complete response [CR], partial response (PR), progressive disease [PD], and stable disease [SD]). The differences between immunotherapy response criteria lie principally in the way measurable lesions are measured (uni- or bi-dimensional measurements based on RECIST or WHO criteria, respectively), the definition of new lesions (particularly when non-measurable) within the category of PD, and the inclusion of new lesions in the index tumor burden (Table 1).
Comparison of the Different Radiological Response Criteria.
| RECIST 1.1 | irRC | irRECIST | iRECIST | imRECIST | |
|---|---|---|---|---|---|
| Number of target lesions | Two per organ (5 in total) | Five per organ (up to 10 visceral and 5 skin lesions) | Two per organ (5 in total) | Two per organ (5 in total) | Two per organ (5 in total) |
| Measurable size and dimension | Solid organ lesions: 10mm (largest diameter). Lymphadenopathies: 15mm (smallest diameter)One-dimensional | ≥5×5mmTwo-dimensional | Solid organ lesions: 10mm (largest diameter). Lymphadenopathies: 15mm (smallest diameter)One-dimensional | Solid organ lesions: 10mm (largest diameter). Lymphadenopathies: 15mm (smallest diameter)One-dimensional | Solid organ lesions: 10mm (largest diameter). Lymphadenopathies: 15mm (smallest diameter)One-dimensional |
| Tumor burden | SLD of target lesions | Sum of the products of the 2 largest diameters (perpendicular to each other) of the target lesions | SLD of target lesions | SLD of target lesions | SLD of target lesions |
| Non-measurable lesions | 1. Non-measurable lesions not selected as target lesions.2. Unmeasurable disease, including lesions that are too small (<10mm for solid organ lesions or <15mm for lymphadenopathies)3. Other lesions difficult to measure in a reproducible manner* | Not defined. Non-target lesions include carcinomatous lymphangitis, skin involvement of breast cancer, and palpable but unmeasurable abdominal masses | As in RECIST 1.1 | As in RECIST 1.1 | As in RECIST 1.1 |
| Appearance of new lesions | The appearance of new measurable lesions implies PDThe unequivocal progression of new non-measurable lesions implies PD | The appearance of new lesions does not necessarily imply PD, and they are included in the total tumor burdenThe appearance of new non-measurable lesions does not imply progression but precludes CR | The appearance of new lesions does not necessarily imply PD and they are included in the total measured tumor burdenThe unequivocal progression of new non-measurable lesions implies PD (irPD) | The appearance of new measurable lesions is assessed according to RECIST 1.1 criteria but recorded separately and are not included in the SLD of target lesions identified in the baseline study. The appearance of new measurable lesions is defined as unconfirmed progression (“iUPD”); This progression is confirmed (“iCPD”) only if additional new lesions appear in subsequent studies or if an increase in the size of previous new lesions is demonstrated (≥5mm for the sum of new target lesions or any increase in new non-target lesions)The appearance of new non-measurable lesions is recorded but not included in the tumor burden; they imply iUPD. To confirm progression, an increase in the size or number of such lesions must be demonstrated within 4–8 weeks. | The appearance of new measurable lesions does not necessarily imply PD, and they are included in the total tumor burdenThe appearance of new non-measurable lesions does not imply progression but precludes CR |
| Evaluation of response | CR: Complete resolution of lesions (lympadenopathies must have a diameter [short diameter] <10mm) | CR (irCR): Complete disappearance of all lesions (which must be confirmed ≥4 weeks from the first finding of disappearance) | CR: Complete disappearance of all lesions (measurable and non-measurable); lymphadenopathies must have a diameter [short diameter] <10mm. Confirmation is not required | CR (iCR): Complete resolution of all lesions (confirmed ≥4 weeks) | CR: Complete disappearance of all lesions (which must be confirmed ≥4 weeks from the first finding of disappearance) |
| PR: Tumor burden decreased by ≥30% | PR (irPR): Tumor burden decreased by ≥50% compared to baseline study (which should be confirmed ≥4 weeks from first finding) | PR (irPR): Total measured tumor burden decreased by ≥30% compared to baseline. Non-target lesions are considered irNN (neither complete response [irCR] nor progression [irPD]). New non-measurable lesions should not reflect unequivocal progression | PR (iPR): Tumor burden decreased ≥30% from baseline (in the absence of new-onset lesions or progression of non-target lesions) | PR: Tumor burden decreased by ≥30% from baseline (in the absence of new-onset lesions or progression of non-target lesions) | |
| SD: Does not meet CR, PR, or PD criteria | SD (irSD): Does not meet irCR or irPR criteria, in the absence of irPD | SD (irSD): Does not meet irCR or irPR criteria, in the absence of irPD | SD (iSD): Does not meet PR or PD criteria in the absence of new lesions or progression of non-target lesions | SD: Does not meet PR or PD criteria in the absence of new lesions or progression of non-target lesions | |
| PD: Defined as an increase of ≥20% in SLD (provided that this absolute increase is ≥5mm) with respect to nadir (best response); unequivocal progression of non-target lesions; or the appearance of new lesions. Confirmation of PD is not required and the patient's clinical situation is not taken into account | PD (irPD): Defined only from measurable disease. Progression is not confirmed if a status of “no progression” is reached (beyond 4 weeks from PD finding). PD is reached when the sum of the products of the 2 largest perpendicular diameters is ≥25% (with respect to baseline/nadir study) | PD (irPD): Defined as an increase of ≥20% (with an absolute increase ≥5mm) of the total measured tumor burden with respect to nadir (or irPD due to non-target lesions or due to the appearance of new non-measurable lesions). Confirmation of PD is recommended at least 4 weeks after the first finding of irPD | PDiUPD: Tumor burden increased by ≥20% (with respect to nadir), progression of non-target lesions, or the appearance of new lesions. The status of iUPD must be confirmed, which can be achieved by finding an additional increase in size (or number of new lesions) in the category of lesions in which progression was initially detected (target or non-target lesions) or by progression (according to RECIST criteria 1.1) in lesion categories that had not previously met progression criteria (according to RECIST 1.1)Progression in the “target lesion” category is confirmed if the next radiological control after reaching the status of iUPD (4–8 weeks later) confirms an additional increase in the sum of the measurements of the target disease (since the attainment of iUPD, as long as this additional increase is, in absolute terms, at least 5mm). The patient's clinical situation is taken into account when deciding whether to continue treatment once the iUPD status is reachediCPD: Disease progression is confirmed (iCPD) if the subsequent radiological control (performed 4–8 weeks after iUPD) shows additional new lesions, a progressive increase in the size of new lesions previously detected (an increase in the sum of new target lesion diameters of at least 5mm or any increase in new non-target lesions), or a progressive increase in pre-existing target or non-target lesions after iUPD | PD: Defined only from measurable disease. Progression is not confirmed if a status of “no progression” is reached (beyond 4 weeks from PD finding). PD is reached when SLD (RECIST) is ≥20% (with respect to baseline/nadir study) |
CR: complete response; iCPD: immune confirmed progressive disease; iCR: immune complete response; iPR: immune partial response; irCR: immune-related complete response; irNN: non-irCR and non-irPD; irPD: immune-related progressive disease; irPR: immune-related partial response; irSD: immune-related stable disease; iSD: immune stable disease; iUPD: immune unconfirmed progressive disease; PD: progressive disease; PR: partial response; SD: stable disease; SLD: sum of largest diameters.
RECIST 1.1: This version, introduced in 2009, simplified the assessment of therapeutic response with respect to the previous version, provided a better description of the evaluation of adenopathies, and incorporated metabolic imaging techniques such as positron emission tomography. These are the most commonly used criteria for the assessment of therapeutic response in solid tumors and are still the only criteria validated and accepted by the major regulatory agencies.19,22–24
irRC (immune-related response criteria): irRC criteria were introduced in 2009. Their main contribution was the concept of “total tumor burden” by which new measurable lesions are added to index lesions. Another change from conventional criteria is that any PD must be confirmed within a minimum of 4 weeks. The limitations of the irRC include: (1) the fact that it is based on the WHO criteria, preventing comparison with the RECIST criteria when using bi-dimensional measurements; (2) less information is provided on adenopathic disease, and (3) treatment effectiveness may be overestimated, as new non-measurable lesions are not taken in account.25–27
irRECIST 1.1 (immune-related RECIST): These criteria were developed in 2014 with the intention of aligning the irRC criteria with RECIST 1.1. They simplified the assessment of response to immunotherapy, permitting comparisons with trials using RECIST (more reproducible unidimensional measurements). In the irRECIST criteria, new measurable lesions are added to the index lesions in a concept called “total measured tumor burden”. As with the irRC criteria, any PD must be radiologically confirmed within a minimum of 4 weeks.28,29
iRECIST (immune RECIST): These criteria, published in 2017, emerged from a consensus between the RECIST working group, the pharmaceutical industry, and the regulatory agencies, and were intended to standardize and validate response criteria with immunotherapy. They set down consensus guidelines to ensure consistent study design and data collection, and to facilitate the comparative analysis of immunotherapy-based CTs. iRECIST postulates are similar to RECIST 1.1 or irRECIST, but new lesions are recorded separately (not added to the index lesions). A key concept is “immune unconfirmed progressive disease” (iUPD), a category assigned to the first PD finding that must be confirmed within 4–8 weeks.27,30,31
imRECIST (immune-modified RECIST): First described in 2018, these criteria are based on the principles of the irRC criteria and entail a modification of RECIST 1.1. New measurable lesions are added to target index lesions (unlike iRECIST). imRECIST associate response or progression patterns with overall survival using indirect evaluation criteria.32
Atypical Response PatternsIn the conventional response criteria, it was assumed that an increase in tumor burden (or the appearance of new lesions) invariably involved PD and, consequently, treatment was discontinued. However, in patients receiving immunotherapy it was observed that despite an initial/transient increase in tumor burden or the appearance of a new lesion, CR, PR, or SD could be achieved (in some patients).21,33 This atypical response is called pseudoprogression and, according to conventional criteria, would erroneously be classified as PD. Other atypical responses that can be observed with immunotherapy are dissociated or paradoxical responses and hyperprogressions.34–36 It is important to bear these atypical responses in mind in patients with LC, because conventional criteria (e.g. RECIST) may underestimate the benefit of ICI in 11% of cases, since they do not correctly interpret the phenomena of pseudo-progression and dissociated or paradoxical response.37
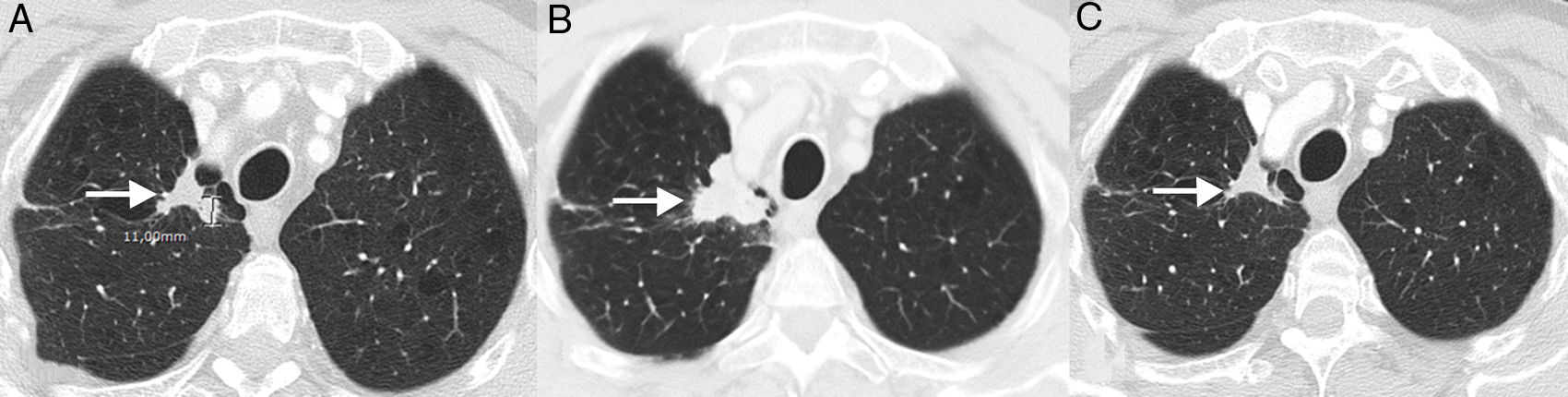
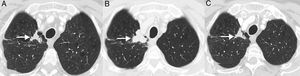
PseudoprogressionPseudoprogression is a well documented phenomenon that is characterized by response or stability following an initial increase in tumor burden or despite the appearance of new lesions in patients treated with immunotherapy.38 It represents false tumor progression, and means that an initial or transient increase in tumor burden (or the appearance of new lesions) on radiology in patients with LC treated with immunotherapy must be confirmed within a certain period of time before “real” disease progression can be assigned and treatment withdrawn (most authors suggest that any progression must be confirmed within 4–8 weeks after first radiological evidence). The underlying mechanism appears to be a result of transient recruitment and infiltration of tumor lesions by inflammatory cells, especially T cells (Fig. 1).39 This phenomenon usually occurs in the first 3 months after starting immunotherapy, although late presentations have been described.40 The incidence of pseudoprogression is variable (0.6%–15.8%) depending on the type of drug (most common with anti-PD-1 agents) and the type of cancer treated (highest incidence in melanoma).41 In LC, however, it affects 0.6%–5.8% of patients, so most LC patients receiving immunotherapy who present initial radiological progression will experience real progression.42 The most important implication of pseudoprogression is that patients obtain clinical benefit and greater survival than patients with true progressions. In a recent study of 160 patients with LC treated with ICI, pseudoprogression was observed in 5%, while all patients obtained clinical benefit.37 Some authors suggest that tumor lesions showing progression should be biopsied to try to differentiate real progression from pseudoprogression. However, even if the presence of inflammatory cells in the biopsy material suggests pseudoprogression, the appropriate time for biopsy has not been defined and the coexistence of tumor and inflammatory cells in the same tissue sample remains to be clarified.43 Moreover, it is not always possible to obtain histological material from all LC patients with suspected pseudoprogression. Patients with pseudoprogression usually do not experience clinical deterioration, so clinical impressions must also be taken into account alongside the radiological and histological data.
Example of pseudoprogression in a 59-year-old patient with progressive non-small cell lung cancer who started second-line immunotherapy (nivolumab). (A) Baseline axial image (pre-immunotherapy) of chest CT (lung parenchyma window) showing a primary tumor lesion in the right pulmonary vertex (arrow). (B) Axial image of chest CT 8 weeks after starting treatment with nivolumab showing radiological progression of the pulmonary lesion (arrow); this apparent tumor progression was not accompanied by clinical worsening, so we decided to continue treatment. (C) Axial chest CT image obtained 6 weeks later, showing a significant reduction in lesion size (arrow), confirming pseudoprogression. According to conventional response criteria (RECIST), treatment would have been interrupted as soon as radiological progression was demonstrated.
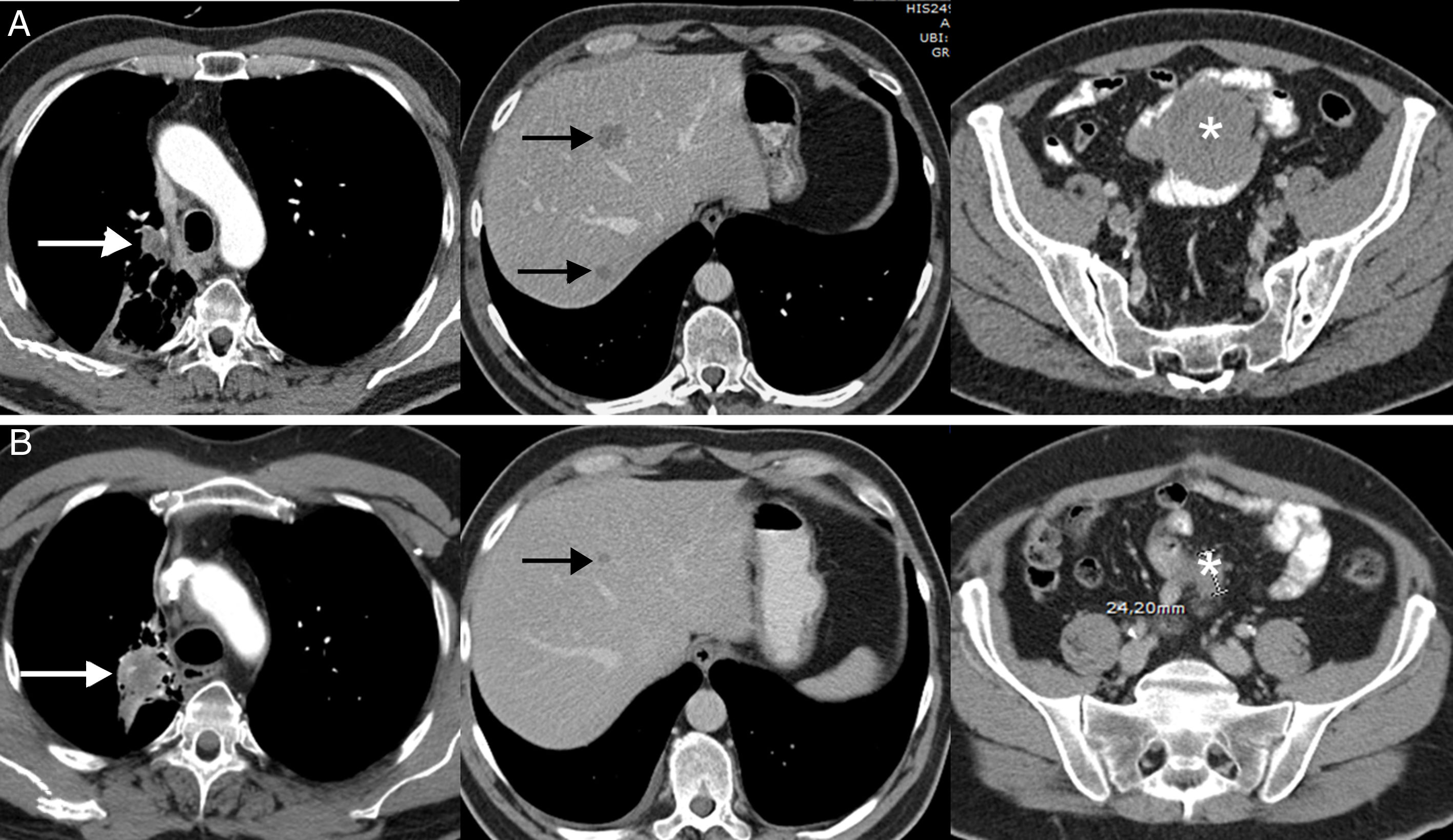
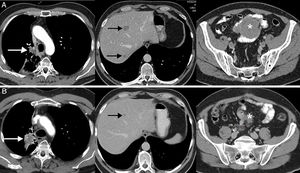
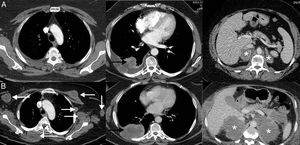
The dissociated or paradoxical response consists of a disparate response of tumor lesions in the same patient, with progression in some lesions and shrinkage in others.36 In patients with LC, both progression of the primary tumor (lung lesion) with favorable response of metastatic lesions and response of primary lesions with progression of secondary lesions have been described.36,37 This phenomenon has not been studied in such depth as pseudoprogression, but also usually appears in the first months after the onset of immunotherapy. The most important implication of the dissociated or paradoxical response is that patients obtain some clinical benefit, although this is less than in patients with pseudoprogression, who usually show longer survival than patients with true progression. In a recent study of 160 patients with LC treated with ICI, pseudoprogression was observed in 8%, half of whom obtained clinical benefit.37 This scenario raises diagnostic and therapeutic dilemmas, since signs of both favorable response and tumor progression may be seen in the same patient44; in some cases, biopsy or local treatment (surgery, stereotactic radiation therapy, etc.) of a progressive tumor lesion may be proposed, although each case requires multidisciplinary evaluation (Fig. 2).
Example of a dissociated/paradoxical response in a 57-year-old patient with progressive non-small cell lung cancer who started second-line immunotherapy (pembrolizumab). The top row (A) shows the baseline CT scan (pre-immunotherapy) while the bottom row (B) shows the CT scan obtained 8 weeks after the start of treatment. (A) Baseline axial CT images (pre-immunotherapy) of chest, abdomen, and pelvis (left to right, respectively) showing primary lung tumor (white arrow), liver metastases (black arrows), and mesenteric mass (asterisk). (B) Axial CT images of the chest, abdomen, and pelvis 8 weeks after starting treatment (left to right, respectively) showing a discrepant response among the lesions: pulmonary progression (white arrow) but favorable response of hepatic (black arrow) and mesenteric metastases (asterisk). Given the patient's good overall condition, we decided to resect the lung lesion, and the patient achieved a complete response in subsequent radiological studies.
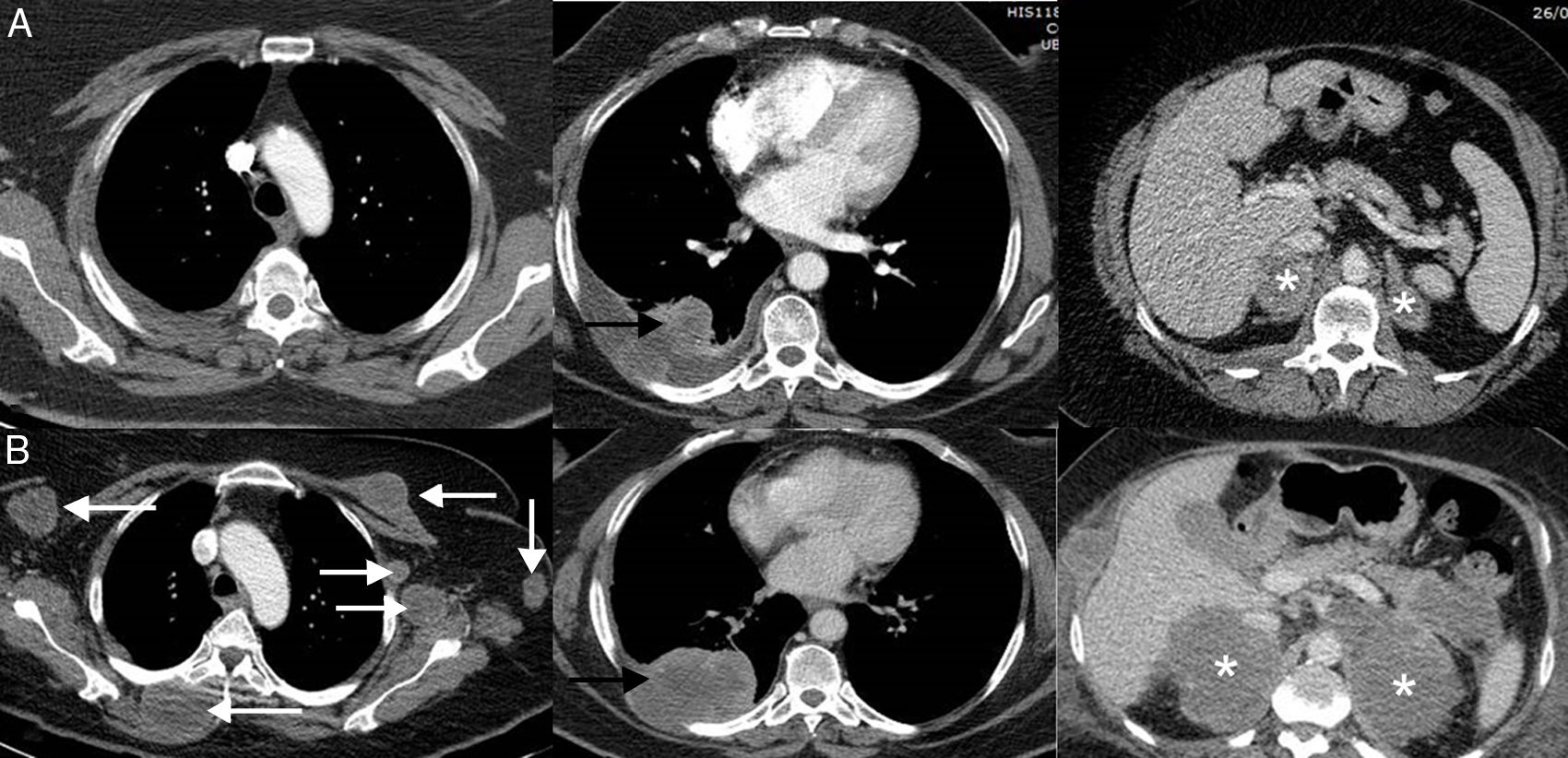
Hyperprogression in a dramatic increase in tumor burden, usually with poor prognosis, in a patient who starts treatment with immunotherapy.45 In hyperprogression, progression after starting immunotherapy treatment is faster than expected or reported for that cancer. This atypical response is not unique to immunotherapy, but it is documented more frequently with this approach than with other therapies (9%–16%).46 Unlike pseudoprogression and dissociated or paradoxical response, the most significant consequence is that patients have much worse survival, and usually die within a few weeks.47,48 In a study of 406 patients with LC treated with ICI, hyperprogression was described in 13.8% of patients, most of whom died within 8 weeks of initiation of immunotherapy treatment.49 Predictors of this grim response are not well defined (Fig. 3).50
Example of hyperprogression in a 73-year-old patient with progressive non-small cell lung cancer who started second-line immunotherapy (pembrolizumab). The top row (A) shows the baseline CT scan (pre-immunotherapy) while the bottom row (B) shows the CT scan obtained 8 weeks after the start of treatment. (A) Baseline axial CT images (pre-immunotherapy) of upper chest, lower chest, and abdomen (left to right, respectively) showing primary lung tumor (black arrow), bilateral supradrenal metastases (asterisks). (B) Axial CT images of upper chest, lower chest and abdomen 8 weeks after starting treatment (left to right, respectively) showing marked progression of known tumor lesions (black arrow and white asterisks), and the appearance of multiple masses in soft tissues and left axilla (white arrows). The patient died 3 weeks later.
Because of their particular mechanism of action, ICIs (“prototype” immunotherapy drugs) are associated with a spectrum of adverse reactions (immune-related adverse events [irAE]) that differ from the adverse effects of cytotoxic chemotherapy.51 These irAEs are attributable to a “proinflammatory” or autoimmune state caused by the indiscriminate enhancement of the immune system (especially T cells) and can affect any organ from the skin and digestive tract to the liver, endocrine glands or lung.52 Some irAEs, such as skin rash, are obvious on physical examination, but others, such as a sarcoid reaction, fasciitis, or fat stranding may be silent and manifest only on radiology studies before they are clinically evident.41,53 Therefore, it is crucial that radiologists detect and report these reactions early, as irAEs are often treated by withdrawing immunotherapy (transitory or definitive, depending on their severity) and adding corticosteroids (or other immunosuppressants).54,55 It is also important that they are identified, given the interesting observation that patients who develop an irAE present better disease control than patients who do not.56 The incidence, severity, and type of irAEs vary according to the type of drug, but range from 54% with anti-CTLA-4 to 14% with anti-PD-L1.57 We will first discuss irAEs affecting the lung parenchyma, because of the potential impact on the interpretation of radiological images in LC patients.
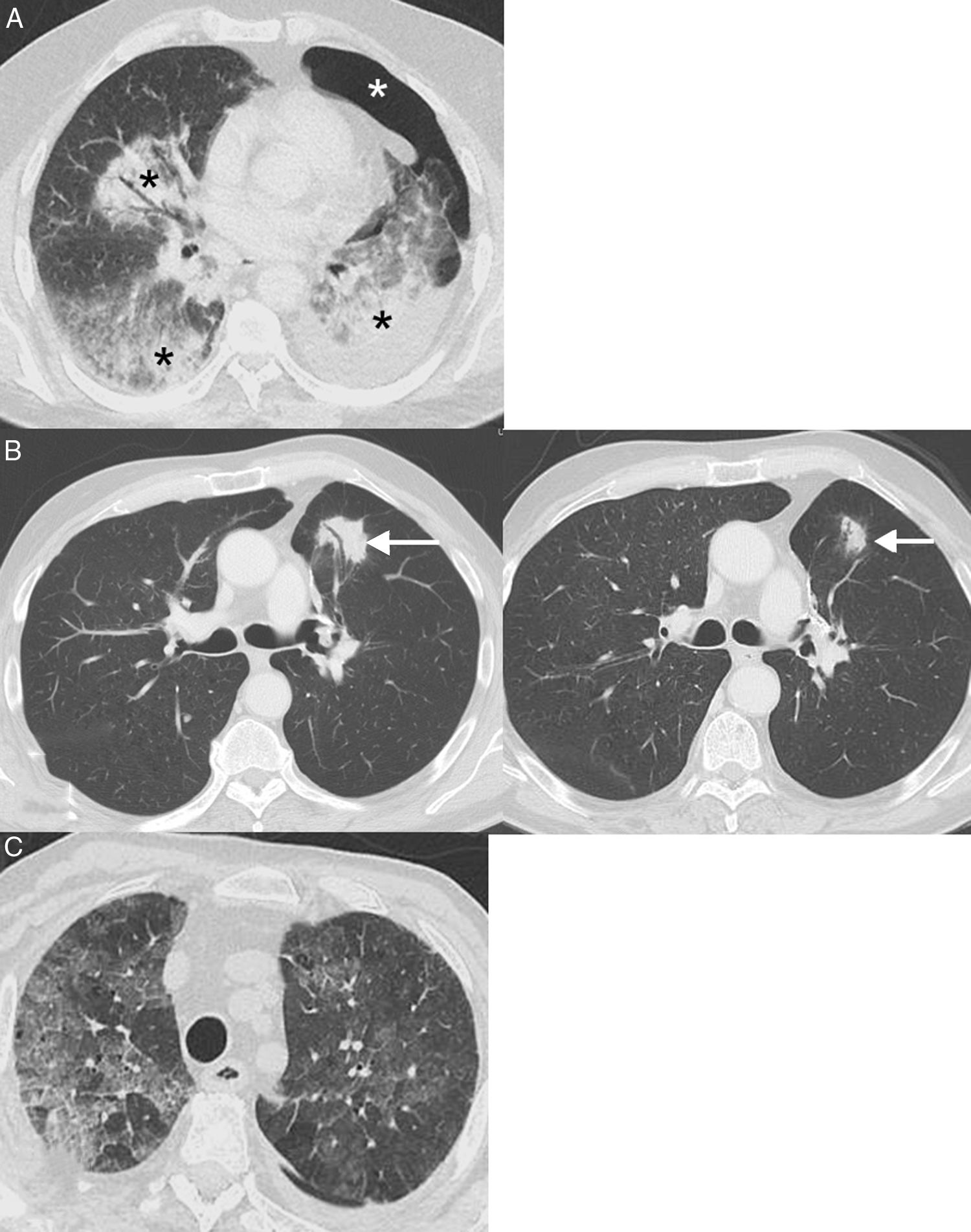
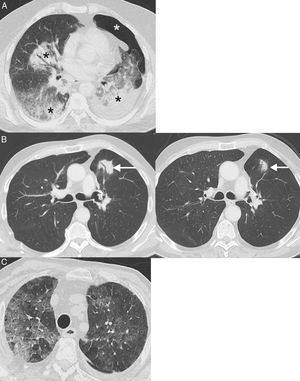
Pneumonitis: This life-threatening irAE has been associated with all types of immunotherapy, although it is more common with PD-1 inhibitors (pembrolizumab and nivolumab) for which incidences of up to 6% are reported.58 A recent meta-analysis showed that pneumonitis occurred more frequently in patients with LC and renal cell carcinoma than in patients with melanoma, within an average of 3.8–7.8 months since administration of the drug.59 Four computed tomography (CT) patterns of ICI pneumonitis have been described: organizing pneumonia (the most frequent, consisting of the appearance of peribronchial consolidations), non-specific interstitial pneumonia (appearance of bilateral ground glass opacities with or without traction bronchiectasis), hypersensitivity pneumonitis (centrolobular nodules with or without mosaic attenuation of the lung parenchyma), and acute interstitial pneumonia (the least common, consisting of bilateral patchy opacities with ground glass attenuation)60 (Fig. 4). In patients with LC, pneumonitis can clinically and radiologically simulate tumor progression or lung infection. The diagnosis is made by combining clinical, analytical, and radiological data, so close multidisciplinary collaboration is essential.61
Examples of pneumonitis (lung toxicity). (A) A 64-year-old patient with metastatic lung cancer receiving immunotherapy (nivolumab) in whom the appearance of bilateral peribronchial consolidations (black asterisks) was observed on CT. In this case, we decided to perform a core needle lung biopsy of a dominant consolidation in the left lower lobe, which showed foci of organizing pneumonia and absence of tumor cells; the biopsy was complicated by left pneumothorax (white asterisk). (B) A 68-year-old patient with locally advanced lung cancer receiving adjuvant immunotherapy (nivolumab); the left CT image shows the appearance of a lesion (arrow) with air bronchogram in the left lung. In this case, we decided to administer empirical treatment with systemic corticosteroids. A repeat chest CT at 4 weeks (right) confirmed partial resolution of pulmonary opacity (arrow). The radiological pattern suggested organizing pneumonia. (C) A 76-year-old patient with metastatic lung cancer receiving immunotherapy (pembrolizumab) who presented in the emergency department with dyspnea. Chest CT showed extensive bilateral ground glass attenuation opacities (pattern of non-specific interstitial pneumonitis). The patient improved after discontinuation of immunotherapy and administration of systemic corticoids.
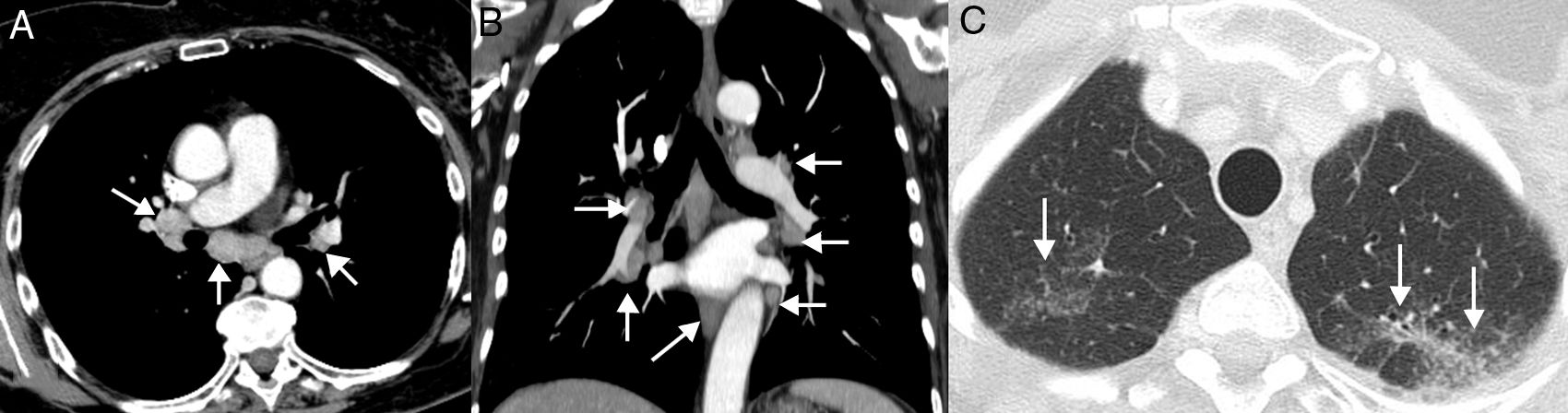
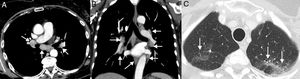
Sarcoid reactions: This is a rare, though important, systemic complication that can mimic PD in LC patients receiving immunotherapy.62 Radiologically, it is characterized by the appearance of lymphadenopathies and small perilymphatic pulmonary nodules, so it can be confused with PD or carcinomatous lymphangitis.63 The demonstration of granulomas is required to confirm diagnosis, although this is not always feasible, so diagnosis is often made by combining clinical and radiological data after ruling out alternative causes (such as infections or tumor progression)64 (Fig. 5). In general, patients with sarcoid reactions present a good overall status with signs of tumor response in other organs, and respond quickly to corticosteroids. A sarcoid reaction, unlike pneumonitis, does not require discontinuation of immunotherapy.65
Example of a sarcoid reaction. A 68-year-old patient diagnosed with metastatic lung cancer receiving immunotherapy (atezolizumab). The patient's clinical status was good and she presented no clinical data of tumor progression, although she complained of a dry cough of several weeks of evolution. During CT follow-up, several lymphadenopathies were observed in the mediastinum and both pulmonary hila (arrows) with a relatively symmetrical distribution (A and B, mediastinum window) and several millimetric pulmonary nodules (C, lung window) with a perilymphatic distribution in the upper lobes (arrows), suggesting a radiological diagnosis of sarcoid reaction. Endobronchial ultrasound confirmed the presence of granulomas and ruled out tumor progression. In this case, we decided to administer corticosteroids, while continuing immunotherapy, and the patient's clinical–radiological picture improved.
Immunotherapy is already part of our therapeutic arsenal for patients with LC, but many challenges remain, one of which is to achieve a correct radiological assessment of the tumor burden by developing unified criteria for the assessment of tumor response. The development of these criteria is complicated by the fact that LC patients may be treated with a combination of systemic therapies (immunotherapy alone or in combination with conventional chemotherapy, antiangiogenic agents or targeted therapies) and locoregional treatments (stereotactic radiation therapy, radio frequency, etc.), so nuclear radiologists and physicians will face increasingly complex interpretative challenges.66 These criteria should take into account not only the size of the lesions but also their volumetric and metabolic/functional characteristics. Important developments in this respect are new radiotracers (other than 18F-fluorodeoxyglucose) that will allow the study of metabolic processes such as cell replication, oxygen consumption and cell death.35 These radiotracers include radioisotope-labeled monoclonal antibodies (which evaluate the expression of PD-1/PD-L1 cell surface markers) and other molecules (labeled with 64Cu or 89Zr) that enable the labeling of T cells.21 They will help identify responders non-invasively before starting immunotherapy (depending on PD-1/PD-L1 expression) or differentiate pseudoprogression from real progression (depending on T cell activation or non-activation).35 Radiomics (a new term used to describe the extraction of advanced quantitative characteristics from CT or magnetic resonance images) may correlate with underlying molecular and genetic characteristics (radiogenomics) and with different tumor phenotypes, assisting in the development of better biomarkers in the future.67–69 As we gradually overcome these challenges, the diagnostic management of patients will improve, probably impacting positively on their survival and quality of life; these new techniques and strategies may even let us hope that the curing of metastatic LC is not just a dream.
Conflict of InterestsPilar Garrido-López provides consulting and advisory services (Roche, MSD, BMS, Boerhinger Ingelheim, Pfizer, Abbvie, Guardant Health, Novartis, Lilly, Astra-Zeneca, Jansen, Sysmex, Blueprint Medicines, Takeda), gives public oral presentations (Takeda, Astra Zeneca, Roche, MSD, BMS, Pfizer, Novartis, Boerhinger Ingelheim, Gilead, Rovi), and receives financial support for clinical trials (Roche, MSD, BMS, Takeda, Lilly, Pfizer, Novartis, Pharmamar, Celgene, Sanofi, GSK, Theradex Oncology, BluePrint Medicines). The other authors state that they have no conflict of interests.
Please cite this article as: Gorospe L, Pacios-Blanco RE, Garrido-López P. Importancia de la imagen en la valoración de la respuesta al tratamiento con inmunoterapia del cáncer de pulmón. Arch Bronconeumol. 2020;56:380–389.