There is insufficient data on the effectiveness of the interfaces used for nasal continuous airway pressure (nCPAP) in newborn infants. Transpulmonary pressure (PTP) calculated from a measured esophageal pressure (Pes) could be used as a surrogate for the pressure transmitted to the distal airways during nCPAP. We aimed to compare the effectiveness of two nasal interfaces, the nasal mask and bi-nasal short prongs, during a relatively brief period of respiratory support by calculated PTP (cPTP) in infants with transient tachypnea of the newborn (TTN).
MethodsNewborns with TTN who needed respiratory assistance with nCPAP were randomized to use either bi-nasal short prongs or a nasal mask. Esophageal pressure measurements were done in order to calculate PTP with either interface. The primary outcome was the cPTP transmitted with each nasal interface. Esophageal pressure measurements were recorded and PTP values were calculated from Pes measurements at the 1st, 6th, 12th and 24th hours in each patient as long as the respiratory support lasted.
ResultsSixty-two newborns with TTN and on nCPAP were randomized into two groups: Group 1 to use bi-nasal short prongs (n: 31) and Group 2 to use a nasal mask (n: 31). Inspiratory and expiratory Pes and cPTP values at the 1st, 6th, 12th and 24th hours were similar with the two interfaces (P<.05).
ConclusionsA nasal mask is similarly effective and safe as bi-nasal short prongs during a brief period of non-invasive respiratory support with nCPAP in late preterm and term neonates with TTN.
No hay datos suficientes sobre la eficacia de las interfaces que se utilizan para la administración de presión positiva continua de la vía aérea por vía nasal (CPAPn) en neonatos. La presión transpulmonar (PL), calculada a partir de la medición de la presión esofágica (Pesof), podría utilizarse como alternativa para medir la presión transmitida a la vía aérea distal durante la CPAPn. Nuestro objetivo fue comparar la eficacia de 2 interfaces nasales, la mascarilla nasal y las cánulas binasales cortas, durante un periodo relativamente corto de soporte respiratorio mediante la PL calculada (PLc) en neonatos con taquipnea transitoria del recién nacido (TTRN).
MétodosLos neonatos con TTRN que requirieron ventilación con CPAPn se aleatorizaron para el uso de cánulas binasales cortas o mascarilla nasal. Se realizaron mediciones de la presión esofágica para calcular la PL con cada interfaz. La variable de resultado fue la PLc transmitida con cada interfaz nasal. Las mediciones de presión esofágica se registraron y los valores de PL se calcularon a partir de las mediciones de la Pesof en las 1.ª, 6.a, 12.a y 24.a horas en cada paciente durante el tiempo que durara la ventilación mecánica.
ResultadosSe aleatorizaron 62 neonatos con TTRN y tratados con CPAPn en 2 grupos: el grupo 1 usó las cánulas binasales cortas (n=31) y el grupo 2 usó la mascarilla nasal (n=31). Los valores inspiratorios y espiratorios de Pesof y PLc en las 1.a, 6.a, 12.a y 24.a horas fueron similares con ambas interfaces (P<0,05).
ConclusionesLa máscara nasal tiene una eficiencia similar a las cánulas binasales cortas durante la administración breve de ventilación mecánica no invasiva mediante CPAPn en neonatos prematuros tardíos y neonatos a término con TTRN.
Transient tachypnea of the neonate (TTN) is diagnosed in approximately 2% of live births, accounting for the majority of respiratory distress cases in term and late preterm babies. Transient tachypnea of the neonate is a benign, self-limited condition characterized by abnormal fluid overload resulting from delayed clearance of fetal lung fluid. Its management is generally supportive and oxygen is usually provided by hood or nasal cannula.1 On the other hand, some newborns with TTN demonstrate increased work of breathing under supplemental oxygen, and cannot maintain their oxygen saturation above 90%. Given its pathophysiology, nasal continuous positive airway pressure (nCPAP) could be a useful support for neonates with TTN.2
Non-invasive ventilation (NIV) strategies have become the standard for respiratory support in spontaneously breathing newborns.3–5 Various nasal interfaces including bi-nasal short prongs, long nasopharyngeal prongs or nasal masks are currently available to provide nCPAP. Bi-nasal short prong devices are known to be more effective than single prongs in reducing the likelihood of short-term adverse outcomes of re-intubation and respiratory failure in newborns.6,7 Although various nasal interfaces are available to provide nCPAP in newborns, only few studies have compared these devices with respect to effectiveness and side effects, including nasal skin breakdown.8–11
Monitoring functional residual capacity and trans-pulmonary pressure (PTP) represents an opportunity to individualize the interpretation of lung mechanics, and guide development of a ventilator strategy tailored to a given patient.12 Esophageal pressure (Pes) has been proposed as a surrogate for pleural pressure and allows the calculation of PTP, and provides a mean to guide the management of ventilated patients.13
Physiological principles of nCPAP are established on the generated continuous distending pressure by the device and the pressure transmitted to the proximal and the distal airways by the nasal interface used. Up to now, clinical studies on nasal interfaces have generally evaluated their efficacy and safety with respect to clinical outcomes.6–11 In this study, we aimed to prove the hypothesis that nasal mask can be as effective as bi-nasal prong which is the most effective interface. For this purpose, Pes and calculated PTP (cPTP) measurement with clinical results were evaluated with both interfaces in infants with TTN who were assisted with nCPAP.
MethodsResearch Design and SettingsThis prospective study was conducted in a single 25 bed neonatal intensive care unit (NICU) in Ankara University School of Medicine Children's Hospital, Ankara, Turkey with approximately 550 annual admissions, between September 2013 and June 2014. The research design of the trial was based on comparative effectiveness, which we thought would enable a direct comparison of the two clinical interventions and help to determine a better clinical approach regarding the choice of nasal interfaces for nasal respiratory support.14
Both the Local Ethics Committee of Ankara University and the Institutional Review Board of the Ministry of Health of the Republic of Turkey approved the study. The study was registered to ClinicalTrials.gov under identifier NCT01989442 and supported by Ankara University Scientific Research Projects fund (13B3330008). Parental informed written consent was obtained for all participants before enrollment.
Patient EnrollmentEach infant admitted to the NICU during the study period was screened for enrollment. Late preterm, early term and full term newborns with TTN that required nCPAP for respiratory distress were enrolled.
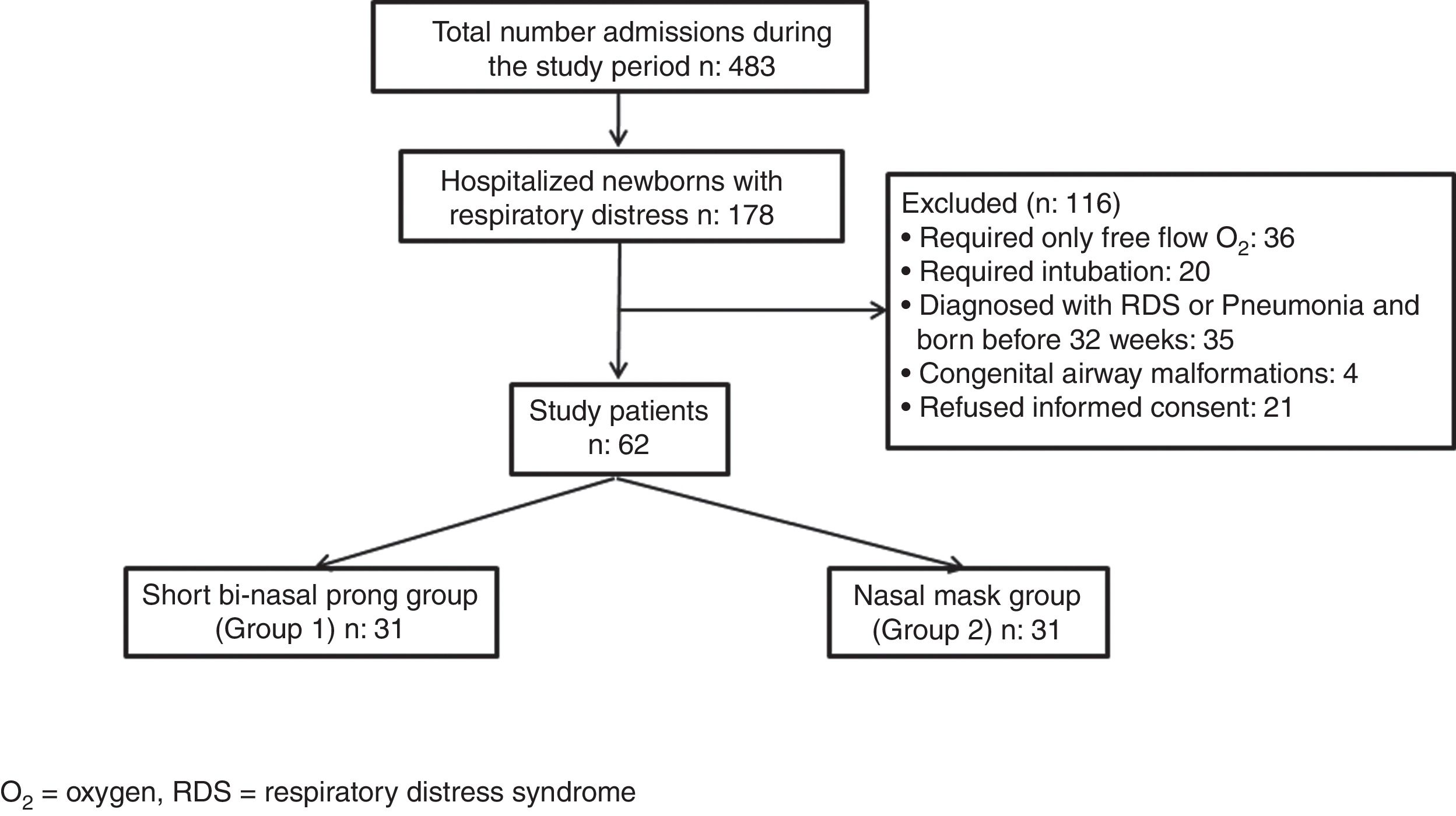
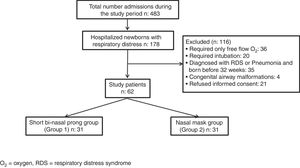
Newborns requiring intubation for respiratory support, preterm infants born before 32 weeks of gestation, infants with major congenital malformations, and other causes of respiratory insufficiency such as respiratory distress syndrome (RDS), meconium aspiration, pneumonia and patients without parental consent were excluded (Fig. 1).
The diagnosis of TTN was established based on the following clinical and laboratory data: (a) onset of tachypnea with a respiratory rate (RR) more than 60breaths/min within 6h after birth, grunting, nasal flaring and retractions; (b) persistence of tachypnea for at least 12h; (c) chest radiograph indicating at least one of the following: prominent central vascular markings, widened pleural fissures, bilateral congestion of the hilus, signs of air trapping; and (d) exclusion of all other respiratory disorders such as RDS, meconium aspiration, congenital heart disease and non-respiratory disorders likely to cause tachypnea (hypocalcemia, persistent hypoglycemia, polycythemia).1
ProceduresEligible newborns were randomly assigned to one of the two experimental groups by using sequentially numbered, sealed, non-transparent envelopes. Patients were randomized to use either bi-nasal short prongs (Group 1) or nasal mask (Group 2) during their respiratory assistance by nCPAP. Nasal masks (Infant Flow LP nasal masks, CareFusion, California, USA) and bi-nasal short prongs (Infant Flow LP nasal prongs, CareFusion, California, USA) were used in the appropriate size according to the body weight and nares scale provided by the manufacturers. Neither silicon tape nor ointment were used between the interface and nose during the study period.
Nasal CPAP was started with fraction of inspired oxygen (FiO2) level of 35% and positive end expiratory pressure (PEEP) level of 6cmH2O when oxygen saturations could not be maintained above 90% accompanied by increased work of breathing, and stopped when oxygen saturations could be maintained above 90% with less than or equal to 5cmH2O nCPAP at less than or equal to 25% FiO2 in each patient.
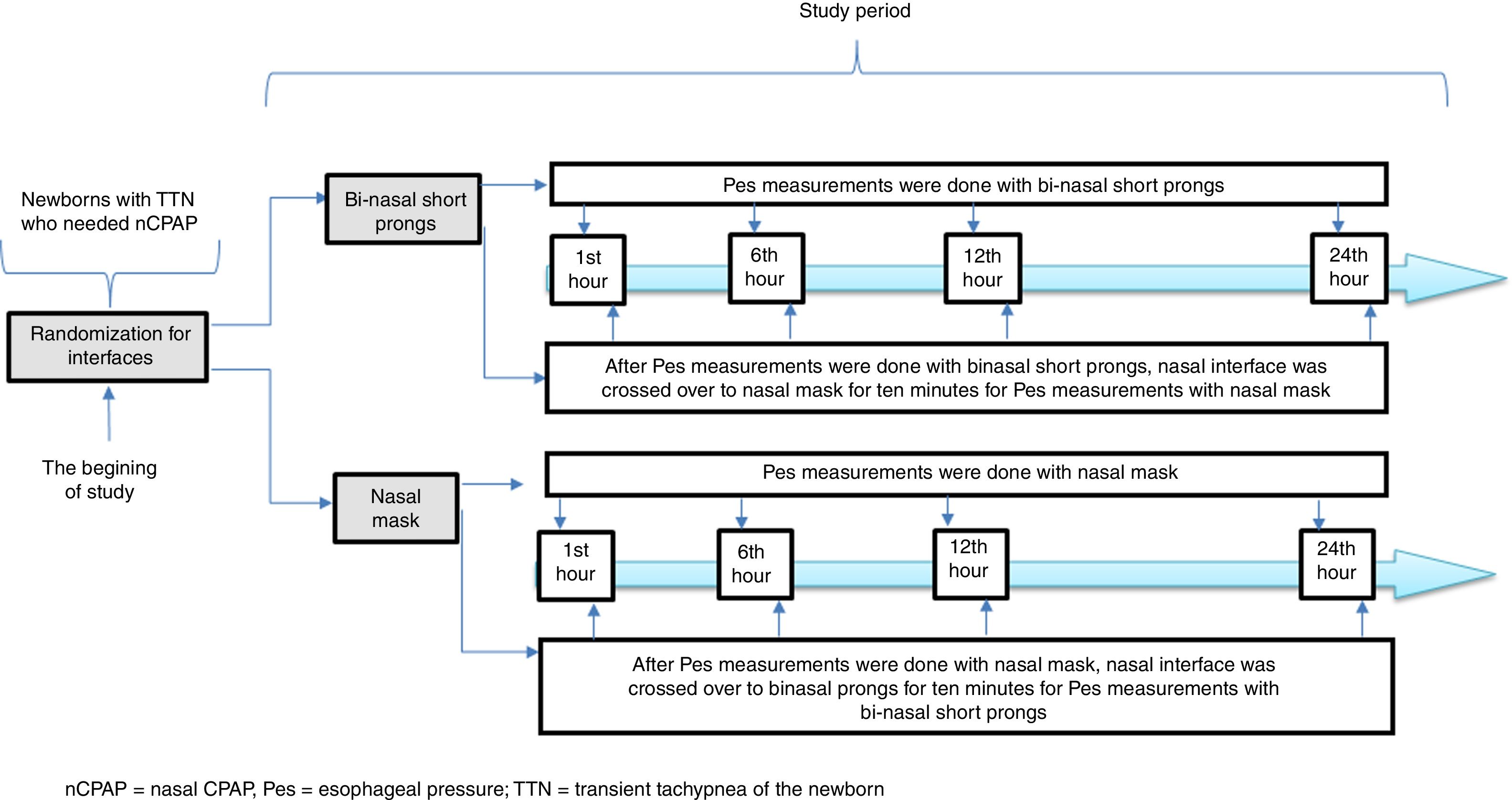
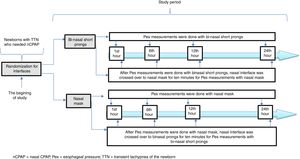
Infant Flow® SiPAP System (VIASYS, CareFusion, California, USA) was used in the nCPAP mode throughout the entire study period. A catheter for esophageal pressure monitoring (Avea SmartCath® esophageal pressure monitoring tube set, transducer, 6 Fr pediatric, CareFusion, New Jersey, USA) was placed via the oral orifice. The location of the Pes monitoring catheter was confirmed by the heartbeats and synchronization of breaths.15 We planned to measure Pes with the alternative interface for each patient in both study groups in order to eliminate the variability due to patient characteristics in the measurement periods. Each interface was crossed over for 10min before the Pes measurements with the alternative interface and the patient was put on the original interface until the next measurement period (Fig. 2). The Pes was recorded separately during the inspiration and expiration periods simultaneous with inspiration and expiration periods of the patient. Esophageal pressure measurements were performed with both interfaces at the 1st, 6th, 12th and 24th hours of respiratory support for each patient. Trans-pulmonary pressure was manually calculated from the equation: PTP=Airway opening pressure (Paw)−Pes. Paw was accepted as the PEEP value in infants on CPAP.15 Esophageal pressure values were obtained from direct measurements. If the patients were off nCPAP due to clinical improvement or NIV failure, they were not included for further assessments. All measurements were made before feedings.
The appropriate interface was used for each patient's weight. In order to determine the effect of nasal trauma due to nasal interfaces, prophylactic nasal care was not applied to any patient prior to nasal injury, and nasal care was started when symptoms of nasal injury appeared. All patients receiving NIV underwent short-term aspiration through orally or nasopharyngeally in cases of increased secretion, cough and desaturation. if needed, FiO2 was temporarily increased during aspiration and restored to its previous settings after suction was completed. Humidification was maintained at appropriate levels to provide physiological functioning of the upper respiratory tract (25–30mg H2O/L) in all patients. To facilitate gas exchange and protect lung tissue, heated inspired gas was maintained at body temperature. Caring protocols were applied to all patients in the same way.
Variables and MeasuresPrimary outcomes were the cPTP, Pes values and any stage nasal trauma. Secondary outcomes were air leaks and the duration of respiratory support with nCPAP and supplemental oxygen. During study period, any barrier was used to avoid nasal trauma. Nasal trauma was staged as mild (stage I), moderate (stage II) and severe (stage III). Mild nasal injury was defined as persistent erythema or non-blanching hyperemia around the nose with intact skin. Moderate injury included bleeding, surficial ulcers or erosions, with partial thickness skin loss. Severe injury comprised excoriation or columella necrosis, with full thickness skin loss.16 Nasal trauma was assessed by the same observer.
Demographic and clinical data related to birth weight (BW), gestational age (GA), antenatal steroid administration, gender, mode of delivery, positive pressure ventilation in the delivery room, Apgar scores at 1st and 5th minutes, PEEP and FiO2 values during the study period were recorded.
Statistical AnalysisA sample size of 62 subjects were estimated to achieve 80% power to detect an intra-class correlation (ICC) of 0.5 under the alternative hypothesis when the ICC under the null hypothesis is 0.00000 using an F-test with a significance level of 0.05.17 Kolmogorov–Smirnov test was used to verify the variable distribution. A comparison between the groups was performed using t-test and/or Mann–Whitney U-test for non-parametric continuous variables in independent-samples and chi-square or Fisher's exact tests as appropriate for categorical variables. The data were presented as mean±standard deviation, and/or median (minimum-maximum) for continuous variables, in addition, percentage and distribution of frequency for categorical variables. Statistical analysis was performed with Statistical Package for Social Sciences (SPSS) version 15 for Windows (SPSS Inc., St. Louis, MO) and statistical significance was set at a two-sided P value of .05.
ResultsDuring the study period, of the 483 infants admitted to the NICU, 178 had respiratory distress, 116 of them were excluded: 36 for requiring only free flow oxygen (O2), 20 for requiring intubation, 35 for being born before 32 weeks of gestation and diagnosed as RDS and/or pneumonia, 4 for having congenital airway malformations and 21 for refused informed consent. Sixty-two of the patients needed nCPAP for TTN and were eligible for the study. They were subsequently randomized to two study groups so as to have 31 infants in each group (Fig. 1).
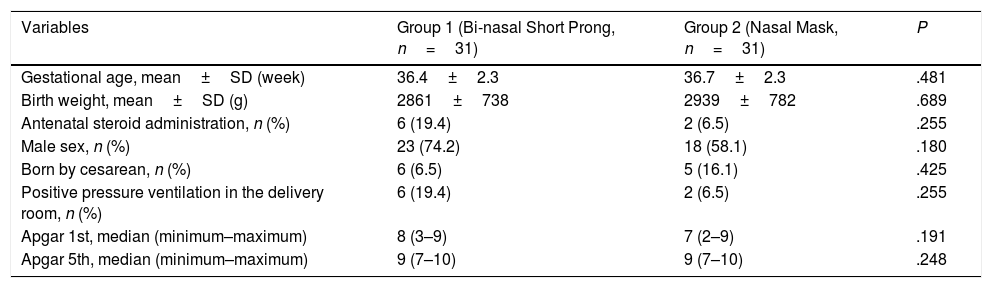
The mean GA and BW of study infants were 36.7±2.31 weeks and 2900±755g, respectively. Male to female ratio was 1.95. The study groups were comparable with respect to GA, BW, gender, being born by cesarean section, having received antenatal steroids and positive pressure ventilation in the delivery room, as well as 1st and 5th minute Apgar scores (Table 1).
Characteristics of the Study Groups.
| Variables | Group 1 (Bi-nasal Short Prong, n=31) | Group 2 (Nasal Mask, n=31) | P |
|---|---|---|---|
| Gestational age, mean±SD (week) | 36.4±2.3 | 36.7±2.3 | .481 |
| Birth weight, mean±SD (g) | 2861±738 | 2939±782 | .689 |
| Antenatal steroid administration, n (%) | 6 (19.4) | 2 (6.5) | .255 |
| Male sex, n (%) | 23 (74.2) | 18 (58.1) | .180 |
| Born by cesarean, n (%) | 6 (6.5) | 5 (16.1) | .425 |
| Positive pressure ventilation in the delivery room, n (%) | 6 (19.4) | 2 (6.5) | .255 |
| Apgar 1st, median (minimum–maximum) | 8 (3–9) | 7 (2–9) | .191 |
| Apgar 5th, median (minimum–maximum) | 9 (7–10) | 9 (7–10) | .248 |
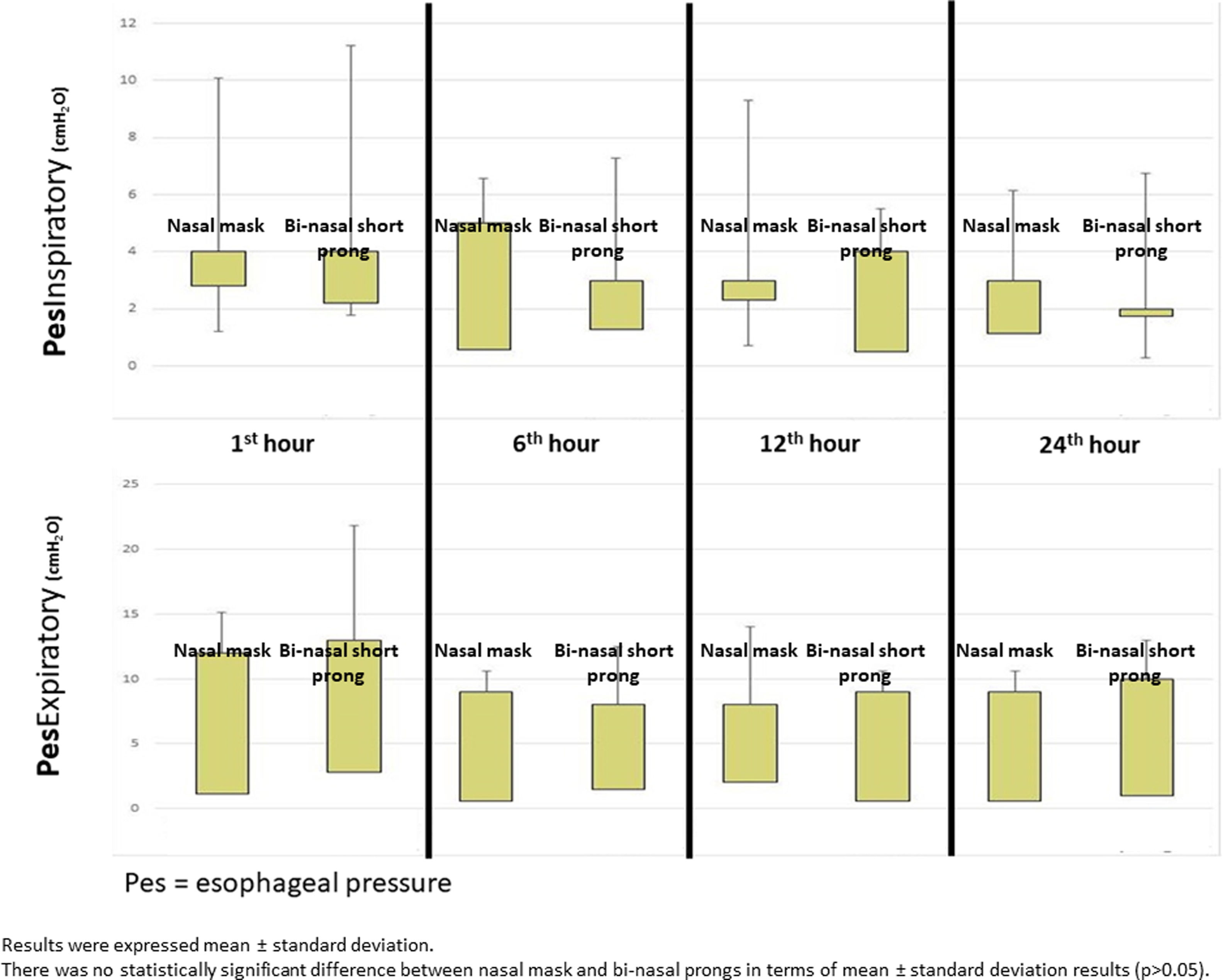
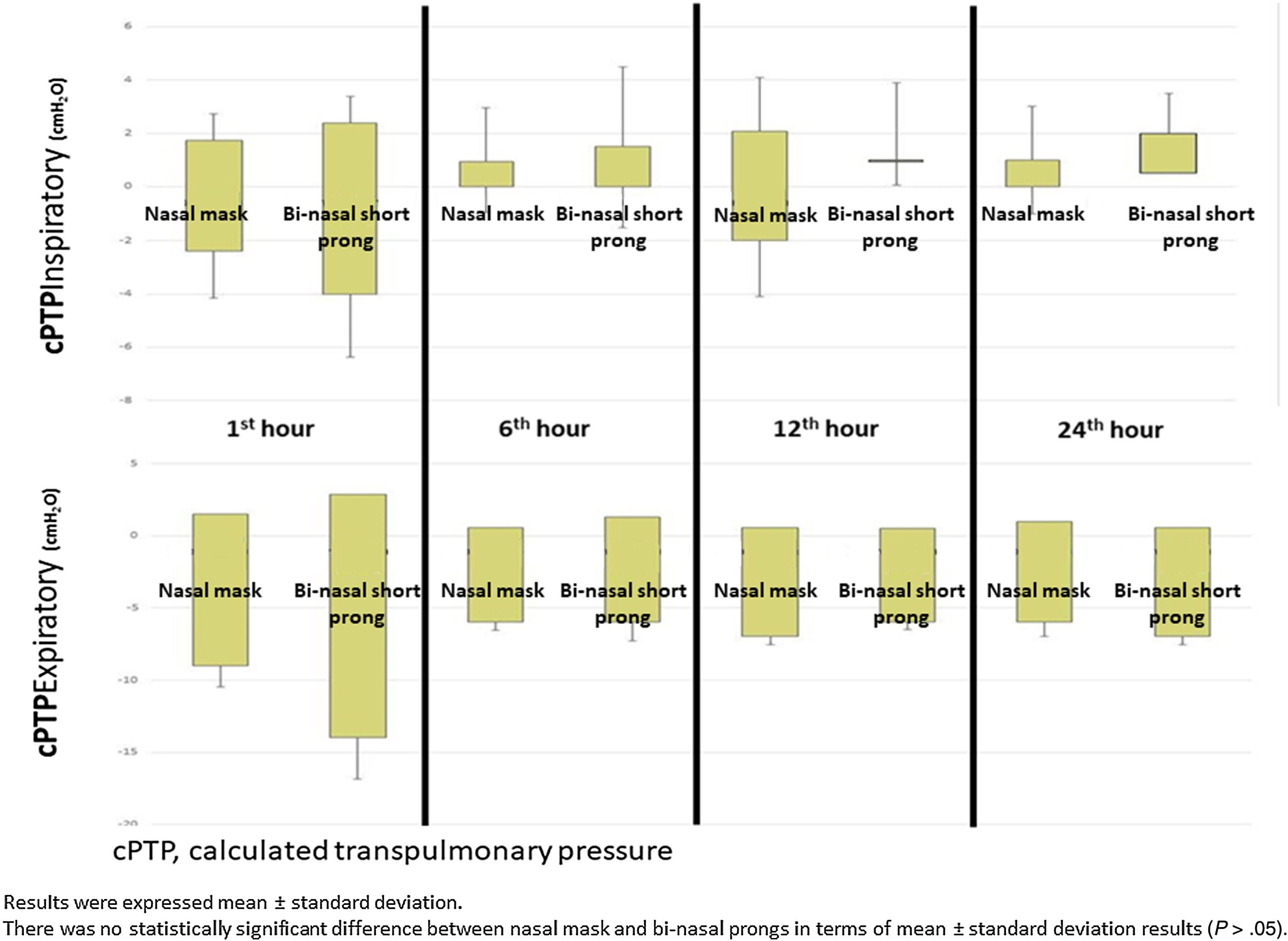
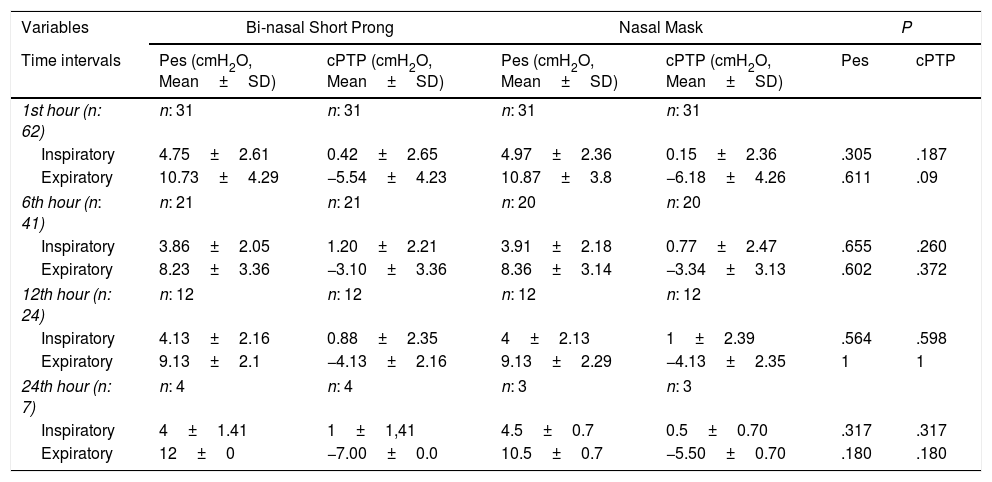
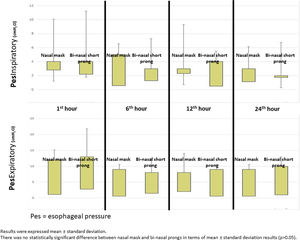
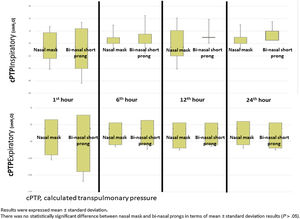
Mean Pes and calculated PTP values with nasal mask and bi-nasal short prongs during inspiratory and expiratory courses of breathing at the 1st, 6th, 12th and 24th hours are given in Table 2. Inspiratory and expiratory Pes (Fig. 3) and cPTP values (Fig. 4) at the 1st, 6th, 12th and 24th hours were similar in the study groups. Applied PEEP and FiO2 values were not significantly different in the study groups at the designated time intervals (Supplementary material 1 Table 3).
Esophageal Pressure and Calculated Transpulmonary Pressure Measurements During Inspiratory and the Following Expiratory Phase at 1st, 6th, 12th, and 24th Hours.
| Variables | Bi-nasal Short Prong | Nasal Mask | P | |||
|---|---|---|---|---|---|---|
| Time intervals | Pes (cmH2O, Mean±SD) | cPTP (cmH2O, Mean±SD) | Pes (cmH2O, Mean±SD) | cPTP (cmH2O, Mean±SD) | Pes | cPTP |
| 1st hour (n: 62) | n: 31 | n: 31 | n: 31 | n: 31 | ||
| Inspiratory | 4.75±2.61 | 0.42±2.65 | 4.97±2.36 | 0.15±2.36 | .305 | .187 |
| Expiratory | 10.73±4.29 | −5.54±4.23 | 10.87±3.8 | −6.18±4.26 | .611 | .09 |
| 6th hour (n: 41) | n: 21 | n: 21 | n: 20 | n: 20 | ||
| Inspiratory | 3.86±2.05 | 1.20±2.21 | 3.91±2.18 | 0.77±2.47 | .655 | .260 |
| Expiratory | 8.23±3.36 | −3.10±3.36 | 8.36±3.14 | −3.34±3.13 | .602 | .372 |
| 12th hour (n: 24) | n: 12 | n: 12 | n: 12 | n: 12 | ||
| Inspiratory | 4.13±2.16 | 0.88±2.35 | 4±2.13 | 1±2.39 | .564 | .598 |
| Expiratory | 9.13±2.1 | −4.13±2.16 | 9.13±2.29 | −4.13±2.35 | 1 | 1 |
| 24th hour (n: 7) | n: 4 | n: 4 | n: 3 | n: 3 | ||
| Inspiratory | 4±1.41 | 1±1,41 | 4.5±0.7 | 0.5±0.70 | .317 | .317 |
| Expiratory | 12±0 | −7.00±0.0 | 10.5±0.7 | −5.50±0.70 | .180 | .180 |
cPTP=calculated transpulmonary pressure; Pes=esophageal pressure; TTN=transient tachypnea of the newborn.
The incidence of nasal trauma was 6.4% in the entire study group. Although the incidence of any stage nasal trauma was higher in the bi-nasal short prong group, the difference between groups was insignificant (P=.113). Four patients with nasal trauma in the bi-nasal prong group had only mild (Stage I) injury. Durations of oxygen supplementation and nCPAP were similar. Pneumothorax requiring a chest tube developed in one patient in the bi-nasal prong group, and one patient in the nasal mask group had pneumomediastinum (Supplementary material 2 Table 4).
DiscussionOur data demonstrates that a nasal mask is similarly effective as bi-nasal short prongs during a brief period of respiratory support by nCPAP in late preterm and term neonates. Pes and cPTP values during both the inspiratory and expiratory phases of breathing at the 1st, 6th, 12th and 24th hours were similar in patients who were treated with nCPAP via nasal mask and bi-nasal short prongs at similarly applied PEEP and FiO2. Nasal injury was insignificantly less with the nasal mask. Respiratory care was performed as standard in all patients treated with NIV in both nasal mask and bi-nasal prong groups. Standard respiratory care and similar PEEP were applied to each patient in both groups, and cPTP and Pes pressures were determined to be similar in the same patient treated with different interfaces. PEEP values between groups were found to be similar. In the same patient, cPTP and Pes values were measured similarly at both interfaces. Visual differences in the distributions seen in Figs. 3 and 4 were given as mean±standard deviation, and the measured values were not statistically different. These results showed that there was no difference in similar PEEP pressure in terms of cPTP and Pes pressures measured in the lungs via both interfaces. Our results showed that nasal mask was as effective as bi-nasal prong. Unlike the studies comparing the efficacy of the two interfaces, our study was the first to evaluate clinical outcomes as well as lung pressure.
There is insufficient data on the effectiveness of the interfaces for nCPAP in late preterm and term infants in the literature. Although a meta-analysis showed that bi-nasal short prong devices were more effective than single prongs in reducing the rate of re-intubation in preterm infants, there is only scant information on nasal mask application.7 Kieran et al. reported that nCPAP with a nasal mask was more effective in prevention of intubation and mechanical ventilation within 72h of starting therapy than bi-nasal short prongs in preterm infants (52% vs. 28%, P=.007).10 The effectiveness of nCPAP via nasal mask was evaluated with respect to clinical outcomes in their preterm population. Our study groups mostly comprised late preterm and early term infants with TTN. Due to the generally benign and self-limited nature of TTN, the number of patients who needed respiratory support with nCPAP was small, the duration of nCPAP was short and the probability of either intubation or nasal trauma was low. Therefore our primary aim was to evaluate the efficacy of transmission of positive pressure to distal airways via either nasal interface.
Esophageal pressure measures pleural pressure in the lung surrounding the esophagus reliably. It may therefore underestimate pleural pressure of the dependent regions and overestimate pleural pressure of the nondependent zones.15 It has been proposed as a surrogate for pleural pressure that allows the calculation of PTP. Pleural pressure ranges widely and unpredictably in elderly patients with acute respiratory distress, due to factors such as obesity and abdominal fluid accumulation, which influence the mechanical behavior of the chest wall.13 It is well accepted that the respiratory changes in Pes are representative of changes in pleural pressure applied to the lung surface.13,18 Esophageal pressure can be used to estimate the PTP during static maneuvers, as a guide to setting PEEP in the adult studies.13 Lavizzari et al. used Pes and PTP in infants with RDS for lung mechanics to compare the efficacy of heated, humidified, high flow nasal cannula and nCPAP. They concluded that both methods provided similar effects on pleural pressure estimated by esophageal pressure.19 Although the results of only a very few studies appear encouraging, data fostering the clinical usefulness of Pes to support decision-making during acute respiratory distress are very limited.15 We used Pes measurements with two different interfaces to determine the effect of each interface on PTP values in the same patient. When the nasal mask was on, expiratory cPTP yielded a statistically insignificant but lower pressure value than bi-nasal short prongs. We can speculate that the work of breathing during the expiratory phase of breathing might be less with a nasal mask.
Assessment of PTP is a minimally invasive technique that allows better assessment of the physiological effects of two separate modes of respiratory support on lung mechanics.15 We used Pes and calculated PTP as surrogates for the pressure transmitted to the proximal and distal airways by two different interfaces used for the same mode of respiratory support (nCPAP), and compared their effectiveness. We found that Pes and PTP were similar with nasal and short bi-nasal prongs during a brief period of respiratory support with nCPAP in infants with TTN. We can conclude that the nasal mask is similarly effective as short bi-nasal prongs in transmitting the pressure generated by the nCPAP device in late preterm and term newborns. To our knowledge, this is the first study performed using Pes and PTP for comparing the effectiveness of nasal interfaces used for non-invasive respiratory support.
The incidence of skin breakdown associated with nasal CPAP in the neonate is high and has been reported to be between 20 and 60%.11 Type of the nasal interface, incorrect sizing and positioning of the prongs, length of therapy, ambient humidity and temperature are the most commonly reported risk factors for nasal injury. Specifically smaller infants, who are prone to longer duration of respiratory support are at the greatest risk.11,20,21 The onset of injury to the nasal columella has been reported to occur within a mean of 2–3 days after commencement of nCPAP, with some cases occurring as early as 18h after commencement.20,21 Overall incidence of nasal injury was low (6.4%) in our study group. This may be attributed to the brief duration of nCPAP in relatively larger infants with higher gestational age and birth weight. Compared to bi-nasal prongs, the nasal mask has been reported to cause less or equivalent nasal injury.11 Incidence of mild (Stage 1) nasal injury was insignificantly higher with bi-nasal short prongs in our study. Four patients in the bi-nasal prong group and none of the patients in the nasal mask group had nasal injury. This finding cannot enable a conclusion that the nasal mask is safer than bi-nasal prongs, but we can at least conclude that the nasal mask is as safe as bi-nasal prongs.
However, our study has some limitations. First, due to the usual favorable outcome of TTN, the number of subjects for direct measurements decreased at later time intervals. This prevents us from making any conclusions with respect to measurements and nasal injury for the interfaces during prolonged nCPAP. Second, because our patient population consisted of relatively mature newborns, we could not make any conclusions especially for extremely preterm infants who are the primary candidates for long-term respiratory support.
ConclusionsThis is the first study done using esophageal pressure and transpulmonary pressure for comparative effectiveness of nasal interfaces used for respiratory support. Our study demonstrates that nCPAP therapy with a nasal mask is as effective as bi-nasal short prongs in neonates in late preterm and term infants.
FundingThe trial supported by Ankara University Research Fund. Project number: 13B3330008.
Conflict of InterestThe authors declare no conflict of interest.
Prior Abstract Presentation: Pediatric Academic Societies (PAS) 2016 Meeting in Baltimore, Maryland, United States of America. April 30–May 3.