Access to quality spirometry is an essential objective in order to be able to minimize the underdiagnosis of respiratory diseases, especially that are most frequent, such as COPD and asthma. This objective can be reached in the short term, but it requires the simultaneous integration of different strategies: training of the health-care professionals who perform spirometry, definition of standards for the transmission of the information, technical requirements for acquiring apparatuses and the correct interpretation of the results.
This present study shows the use of standards for the electronic exchange of clinical information. In order to normalize the treatment of the data related with spirometry and to enable the exchange of information, we have used the standard CDA R2 (Clinical Document Architecture, Release 2) of HL7 (Health Level Seven), version 3. HL7 is a product by HL7 International, a non-profit organization that deals in the production of standards in the health-care setting in order to facilitate interoperability.
Furthermore, defining these standards is essential for ensuring that they are adopted by spirometer manufacturers. By means of this process, the base is set for facilitating access to spirometry at the health-care level, while at the same time it is a fundamental technical element for designing quality control programs of the explorations.
El acceso a una espirometría de calidad es un objetivo imprescindible para poder minimizar el infradiagnóstico de las enfermedades respiratorias, especialmente en las más frecuentes como la EPOC y el asma. Este objetivo es alcanzable a corto plazo, pero requiere la integración simultánea de estrategias diversas: formación de los profesionales que deben realizar la espirometría, definición de estándares para la transmisión de la información, requerimientos técnicos en las adquisiciones de aparatos y la correcta interpretación de los resultados.
El presente trabajo muestra la utilización de estándares para el intercambio electrónico de información clínica. Para normalizar el tratamiento de los datos relacionados con la espirometría y permitir el intercambio de información se ha utilizado el estándar CDA R2 (Clinical Document Architecture, Release 2) de HL7 (Health Level Seven) versión 3. HL7 es un producto de HL7 international, una organización no lucrativa que se dedica a la producción de estándares en el ámbito de la salud para facilitar la interoperabilidad.
La definición de este estándar, además, es imprescindible para asegurar la adopción del mismo por parte de los fabricantes de espirómetros. Mediante este proceso se ponen las bases para facilitar el acceso a la espirometría desde todos los ámbitos asistenciales y, a su vez, es un elemento técnico fundamental para diseñar los programas de control de calidad de las exploraciones.
More than 150 years ago, John Hutchinson became interested in the value of the volume of air that humans could exchange with the environment.1,2 Since then, spirometry (the systematic and standardized measurement of this capacity to mobilize air) has become a key piece in the detection, diagnosis, prognosis and the follow-up of respiratory diseases. Spirometry does not provide the etiologic diagnosis, but it enables us to observe the physiopathological basis of the process and in this manner to approximate the diagnosis by means of signs which are easy to detect: flow and volume.3
Although spirometry is a non-invasive test that is relatively easy to carry out, several studies give evidence of difficulties to access the test and the problems related with the quality of the data collected. In part, the problems related with spirometry are due to the fact that it is a test that requires complete patient cooperation. The expiration maneuver requires intensity, time and coordination in order to obtain measurable values with clinical significance. In order to achieve this, it is crucial for the professionals performing the test to be properly trained. But, moreover, it has been repeatedly observed that the availability of a spirometer does not guarantee its routine use.4 In addition to its underuse, Monteagudo et al.5 observed the incorrect register of spirometric data and their limited impact on treatment changes over the course of patient follow-up.
Despite everything, quality spirometry is a reachable objective, as has been demonstrated in epidemiological studies with different participating observers.6 Therefore, it is possible to aspire to quality spirometry in all health-care settings and even in non-health-care settings, such as in the homes of patients themselves7 and in pharmacies.8
One of the challenges of all health-care systems is the implementation of strategies that promote the change of clinical practices in accordance with the evidence available. The Health Department Directives (Planes Directores del Departamento de Salud)9 intend to make decisions on strategic planning with technicians in order to improve the service provided. Thus, the Master Plan for Respiratory Diseases (Plan Director de las Enfermedades Respiratorias–PDMAR) was created in 2010, after a prior study of nearly two years, in order to improve the care of patients with respiratory diseases.
Since its inception, the PDMAR considered it as priority to guarantee the access to quality spirometry in all health-care settings. Quality spirometry is an essential tool for dealing with the problem of the underdiagnosis of respiratory diseases, especially chronic obstructive pulmonary disease (COPD).10 A favorable element was the confirmation of the availability of spirometers in practically all primary care centers, but the problems described in the literature were likewise confirmed: little regulated training, rotation of the professionals, underuse of the spirometer and limited systematic quality control, both of the apparatuses as well as of the explorations carried out.
Based on the recommendations of experts, a specific workgroup, contributions of scientific societies and the evidence in the literature, these initial proposals for quality spirometry have been defined:
- •
From the outset, attempts made at promoting quality spirometry should be a group effort based on the participation of all the professionals involved, both from the specialized setting as well as from primary care.
- •
The training of the health-care professionals that perform spirometries is crucial and should be accompanied by management policies for the workplace that avoid excessive rotation.
- •
Quality control for spirometry implicates the standardization of the values obtained and the systematic analysis of the explorations. Both elements require communication technologies and guaranteed interoperability among the different apparatuses and the information systems of all the health-care settings.
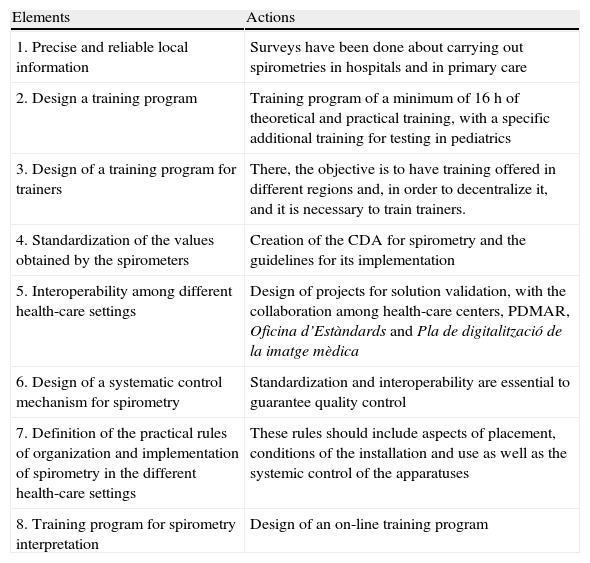
Table 1 summarizes the actions that PDMAR promotes with the aim of providing quality spirometry.
Key Elements for Achieving Quality Spirometry.
| Elements | Actions |
| 1. Precise and reliable local information | Surveys have been done about carrying out spirometries in hospitals and in primary care |
| 2. Design a training program | Training program of a minimum of 16h of theoretical and practical training, with a specific additional training for testing in pediatrics |
| 3. Design of a training program for trainers | There, the objective is to have training offered in different regions and, in order to decentralize it, and it is necessary to train trainers. |
| 4. Standardization of the values obtained by the spirometers | Creation of the CDA for spirometry and the guidelines for its implementation |
| 5. Interoperability among different health-care settings | Design of projects for solution validation, with the collaboration among health-care centers, PDMAR, Oficina d’Estàndards and Pla de digitalització de la imatge mèdica |
| 6. Design of a systematic control mechanism for spirometry | Standardization and interoperability are essential to guarantee quality control |
| 7. Definition of the practical rules of organization and implementation of spirometry in the different health-care settings | These rules should include aspects of placement, conditions of the installation and use as well as the systemic control of the apparatuses |
| 8. Training program for spirometry interpretation | Design of an on-line training program |
Promoted by the Office for Standards and Interoperability of the Catalonian Department of Health (Oficina de Estándares e Interoperabilidad de TicSalut, Departamento de Salud) and by the Plan for the Digitalization of Medical Images (Plan para la Digitalización de la Imagen Médica del Departamento de Salud de la Generalitat de Cataluña), standards have been created in order to achieve the normalization of a complete group of data related with spirometry. These standards have been based on version 3 of HL7, CDA R2. The CDA R2 (Clinical Document Architecture, Release 2) standard by HL7 (Health Level Seven) (version 3) is a product of HL7 International, a non-profitable organization that is dedicated to the production of standards in health care in order to facilitate interoperability. CDA11 defines the structure of a clinical document and uses XML to label the different categories of information. XML is the standard for the structured information exchange between applications, regardless of the technological platform used. A CDA can be viewed from any computer using a web navigator. The CDA for spirometry includes the data of the patient, the information of the context of the test, the resulting clinical parameters, the flow-volume and volume-time charts as well as the original signal captured by the spirometer. Likewise, it includes data about the origin of the request obtained from the electronic medical files of the hospital or the health-care center. The clinical information is coded using SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms), which allows it to be processed automatically and incorporated into the patient medical files in a structured manner.
InteroperabilityThe Office for Standards and the Plan for Digitalization of the Department of Health have elaborated the guidelines for the implementation of the spirometry CDA in collaboration with the Master Plan for Respiratory Diseases (PDMAR).
The availability of the CDA for spirometry allows for information to be exchanged in a standard manner, making the interoperability between different health-care providers and settings possible, executing exploitation services with the data from the resulting standardized spirometry report (very important for research processes) and facilitating the implementation of systematic control processes that guarantee the objective of obtaining quality spirometry.
The suitability of this standard as a mechanism for the exchange of information will be evaluated in various projects with the participation of different service providers and professionals of different health-care settings.
Spirometer RequirementsThe inclusion of this CDA as a technical requirement for acquiring spirometers is essential for ensuring that it is adopted by the manufacturers and for its mid-term generalized use. The Office for Standards has likewise established a procedure for homologation, through which the manufacturers can certify that their equipment conforms to this standard proposed.
The Plan for the Digitalization of Medical Images has proposed to the Catalonian Health Administration (Servicio Catalán de la Salut–CatSalut) the requirements that the spirometers should meet to guarantee interoperability. Table 2 describes the requirements as they appear in the public tender for the acquisition of spirometries. These requirements, which meet a general consensus, are essential to achieve the objectives set by the PDMAR for access to quality spirometry.
Requirements of CatSalut for Spirometries and Calibration Syringes (2011).
| Definition |
| Spirometer with microprocessor and printer for lung function and bronchodilation tests, with memory for storing tests |
| Basic characteristics |
| • Provides spirometry testing in both adults and children |
| • Has graphic incentives |
| • Provides volume-time or flow-time charts of a complete breathing cycle, both forced as well as slow |
| • Provides real-time viewing of the flow/volume and/or volume/time curves on a screen during forced spirometry |
| • Provides the following parameters: |
| - VC |
| - FVC |
| - FEV1 |
| - PEF |
| - FEF25%–75% |
| - FEF75%50%I25% |
| • Memorizes the results from a minimum of 6 tests (6 baseline tests and 6 post-bronchodilation tests) |
| • Provides the possibility for add-ons, such as SaO2modules, etc. |
| • Has exterior finishes that are chip-resistant, hard and color-fast |
| • Incorporates antibacterial filters |
| • Adapted for electric power supply and/or rechargeable batteries |
| • Includes printer for curves and parameters |
| • Exports data in a structured format; all information necessary should be provided in order to integrate these data with the health-care facility's information systems |
| Other characteristics to consider |
| • Interoperable with e-CAP systems (electronic clinical history) |
| • Integrated with the HIS of the centers by means of HL7 messaging in order to receive the activity program |
| • Exports data in the standard format of the Department of Health (CDA R2 for spirometry) |
| • Has Encapsulated CDA Storage, SOP Class 1.2.840.10008.5.1.4.1.1.104.2 |
| • Provides the necessary software, server or client licensing to meet the interoperability requirements as well as the installation and configuration of the software included. In cases when a central server is necessary, it will not be included in the proposal |
| • Saves the different tests of a patient in order to choose the best |
| • Low-cost disposable parts and maintenance |
| • Low risk for contamination; maximum simplicity for sterilization and disinfection of the respiratory circuit; possibility for a disposable transducer |
| • Ports for RS-232 and/or USB, ethernet and data exportation to PC and printer |
| Accessories |
| Basic |
| • Case for the equipment |
| • 3-L calibration syringe |
| Regulations |
| Mandatory |
| • Meets current legislation and other applicable regulations |
In short, the final objective of quality spirometry requires the integration of several strategies: training, definition of standards for the transmission of the data, technical requirements for the acquisition of spirometers, interpretation of results, etc. Each one of these strategies is fundamental, but their isolated impact is minimal if they are not properly integrated. All these should be done in response to a key health-care challenge: early diagnosis of respiratory diseases, particularly the most prevalent such as COPD and asthma. The dissemination of quality spirometry is a goal that is within our grasp in the near future. The previously explained technical requirements will allow us to make forced spirometry a reliable procedure, giving access the numerical as well as charted data, and quality spirometry will become compatible with its extensive use in all health-care settings.
Quality spirometry is a reachable objective.12 The technological elements related with standardization and interoperability are the foundation on which quality spirometry is based. In the first place, without standardization and interoperability, it is very difficult to exchange data among providers with different computing platforms. Furthermore, by means of these technical elements, it is possible to propose performing spirometry outside the health-care system setting, for instance in patients’ homes. But the most important challenge is quality control. All these technological advances should enable routine quality control of the procedures that are performed and, consequently, allow room for improvement. In any given territory, the final objective should be to guarantee the access of all the clinicians to quality spirometry, regardless of the type of health-care center. It is not a fantasy, as it is already possible with chest radiography or electrocardiography, for example. Technology and an integral, multidisciplinary approach is essential for reaching this goal.
Please cite this article as: Salas T, et al. Requerimientos técnicos de los espirómetros en la estrategia para garantizar el acceso a una espirometría de calidad. Arch Bronconeumol. 2011;47:466–9.