The latest tumor, lymph node and metastasis (TNM) classification by the International Association for the Study of Lung Cancer (IASLC), based on the analysis of patients from all over the world, has incorporated changes in the descriptors, especially those regarding tumor size, while proposing new group staging. A new lymph node map has also been developed with the intention of facilitating the classification of the “N” component. SEPAR recommends using this new classification. As for the procedures recommended for staging, in addition to the generalized use of computed tomography (CT), it points to the role of positron emission tomography (PET) or image fusion methods (PET/CT), which provide a better evaluation of the mediastinum and extrathoracic metastases. Endobronchial ultrasound (EBUS) and esophageal ultrasound (EUS) for obtaining cytohistological samples have been incorporated in the staging algorithm, and it emphasizes the importance of precise re-staging after induction treatment in order to make new therapeutic decisions. Comment is made on the foreseeable incorporation in the near future of molecular staging, and systematic lymph node dissection is recommended with the intention of making a more exact surgical-pathological classification.
La última clasificación tumor, ganglio, metástasis (TNM), elaborada por la Asociación Internacional para el Estudio del Cáncer de Pulmón (IASLC), basada en el análisis de pacientes procedentes de todo el mundo, introduce cambios en los descriptores, especialmente en lo referente al tamaño del tumor, y propone una nueva agrupación de estadios. También ha elaborado un nuevo mapa ganglionar que pretende facilitar la clasificación del componente “N”. SEPAR recomienda utilizar esta nueva clasificación. En cuanto a los procedimientos recomendados para la estadificación, además del uso generalizado de la tomografía computarizada (TC), se señala el papel de la tomografía de emisión de positrones (PET) o los métodos de fusión de imágenes (PET/TC), que permiten una mejor evaluación del mediastino y de las metástasis extratorácicas. Se recomienda la incorporación de la ecobroncoscopia (EBUS) y ultrasonografía esofágica (EUS), para la obtención de muestra citohistológica, en el algoritmo de estadificación y se destaca la importancia de una reestadificiación precisa después del tratamiento de inducción para tomar nuevas decisiones terapéuticas. Se comenta la previsible incorporación en el futuro próximo de la estadificación molecular y se recomienda la disección ganglionar sistemática con vistas a una más exacta clasificación quirúrgico-patológica.
More than 10 years have passed since the last publication of the SEPAR Guidelines on the Diagnosis and Staging of Lung Cancer (LC).1 During these years, new procedures have been incorporated into clinical practice, among these being positron emission tomography (either isolated [PET] or integrated with computed tomography [PET/CT]), and the new endoscopic methods for obtaining samples from lymph nodes or other organs: endobronquial ultrasound (EBUS) and esophageal ultrasound (EUS). In addition, the International Association for the Study of Lung Cancer (IASLC) has recently completed a new edition of the classification according to the degree of anatomical extension (TNM classification) based on the detailed analysis of thousand of patients with LC from very diverse regions of the world.2 This classification is quickly being adopted by practically all the societies interested in the study of LC.
Thus, we believe that it is justified to update the SEPAR recommendations on this important topic, although, due to reasons of space, we will limit ourselves to the aspects of staging, without dealing with the diagnosis itself. For the same reason, we also will not comment on the specific aspects of staging small-cell lung cancer (SCLC), although it should be mentioned that in the latest edition of the TNM classification its application is recommended for this type.
Current Staging of Lung CancerThe anatomical TNM-stage classification provides us with a standardized description of lung tumors, compares results between different clinical studies and groups the patients into stages within which the prognosis and therapeutic strategies are similar. The international staging system of 1997 (5th edition) did not undergo any changes in 2002 (6th edition) and has remained untouched until 2009. It has been widely used in non-small-cell lung cancer (NSCLC) and to a lesser degree in SCLC, but its methodology has been criticized. Said system is based on the analysis of the database of one single institution and geographical region made up of 5319 surgically treated patients. It was constituted between 1975 and 1988, when many of the current imaging techniques or therapies were not yet used. All the tumors had clinical and pathological staging, but none of the descriptors T, N or M had been validated either internally or externally.
2009 IASLC Classification: TNM 7th EditionIn order to update and improve the 6th edition, the IASLC, in agreement with the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC), created an International Staging Committee that retrospectively compiled the data of 100869 patients. They were diagnosed between 1990 and 2000, clinically followed for at least 5 years and came from 45 different sources (registers, clinical assays, surgical and hospital series) in 20 countries. They met sufficient quality criteria so that 68463 were analyzed with NSCLC and 13032 with SCLC; in total, 81495 received the following treatments: surgery 41%, chemotherapy 23%, radiotherapy 11%, and 25%, a combination of the three. The findings of the study that could constitute recommendations to change a T, N or M component were validated internally (by geographical region and type of database) and externally with patients from the Surveillance, Epidemiology and End Results (SEER) register from the United States.
Non-small-cell Lung CancerT ComponentThe patients without metastasis were evaluated and, although there was information about different aspects of the T component, only tumor size, existence of accompanying nodules and pleural dissemination could be analyzed in detail.3 The prognostic value of the tumor size was studied in patients with completely resected pathological T1 and T2 N0 M0 tumors who had not received adjuvant therapy. The statistical calculations determined three cut-points at 2, 5 and 7cm, which, in addition to the 3cm, the border between T1 and T2, gave rise to 5 groups of tumors with significantly worse survival with larger tumor diameters. The groups and their 5-year survival rates were: T1≤2cm, 77%; T1>2cm and ≤3cm, 71%; T2>3cm and ≤5cm, 58%; T2>5cm and ≤7cm, 49%, and T2>7cm, 35%. This prognostic gradation was maintained when less selective patient populations were evaluated: clinical staging, incomplete resection and different lymph node affectation. With such arguments, it was decided to subdivide the T1 tumors into T1a (≤2cm) and T1b (>2cm and ≤3cm), and T2 tumors into T2a (>3cm and ≤5cm) and T2b (>5cm and ≤7cm). Likewise, the 5-year survival was compared between patients with T2>7cm tumors and T3 tumors. Similar results were found in the different populations, except in the N0 cases with complete resection, in which it was verified that the survival was even higher in the T3 (41%) than in the T2>7cm (35%), therefore it was decided to reclassify the latter as T3 (Table 1). When we analyzed the tumors that, with pathological staging, presented additional nodules, it was observed that: (a) the 5-year survival of the T3 (31%) was similar to the T4 classified as such due to the existence of an additional nodule or nodules in the same lobe as the primary tumor (28%); (b) the T4 due to other factors had the same survival as those classified as M1 due to an additional nodule(s) in a different homolateral lobe than the primary tumor (22%); and (c) the T4 due to pleural dissemination had a clearly worse prognosis (11% 5-year survival). For the new classification, it was therefore recommended to consider as T3 those tumors with additional nodule(s) in the same lobe as the primary tumor, to consider as T4 those tumors with additional nodule(s) in a homolateral lobe other than that of the primary tumor, and to include in the M category those tumors with pleural dissemination (Table 1).
International Staging System for TNM-stages, 2009 (7th Edition).
| 1. TNM DESCRIPTORS | |
| T (Primary Tumor) | |
| • TX | |
| Primary tumor that cannot be evaluated, or tumor proven by the existence of malignant tumor cells in sputum or bronchial lavage that is not seen by imaging techniques or bronchoscopy | |
| • T0 | |
| No evidence of primary tumor | |
| • Tis | |
| Carcinoma in situ | |
| • T1 | |
| Tumor ≤3cm at the largest diameter, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion proximal to the lobar bronchus (i.e. no invasion of the main bronchus)a | |
| • T1a | Tumor ≤2cm at its largest diameter |
| • T1b | Tumor >2cm but ≤3cm at its largest diameter |
| • T2 | |
| Tumor >3cm but ≤7cm at its largest diameter or tumor with any of the following characteristics (The T2 tumors with these characteristics will be classified as T2a if their diameter is ≤5cm): affects the main bronchus, 2cm or more from the main carina; invades the visceral pleura; associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not affect the entire lung | |
| • T2a | Tumor >3cm but ≤5cm at its largest diameter |
| • T2b | Tumor >5cm but ≤7cm at its largest diameter |
| • T3 | |
| Tumor >7cm or any size that directly invades any of the of the following structures: chest wall (including tumors of the superior sulcus), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or a tumor at less than 2cm form the main carina but not invading it; or associated with atelectasis or obstructive pneumonitis of the entire lung or existence of tumor nodule(s) separated from the primary tumor, in the same lobe | |
| • T4 | |
| Tumor of any size that invades any of the following structures: mediastinum, heart, large blood vessels, trachea, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; or existence of tumor nodule(s) separated from the primary tumor, in a lobe other than the homolateral lung | |
| N (regional lymph nodes) | |
| • NX | |
| The regional lymph nodes cannot be evaluated | |
| • N0 | |
| There are no regional lymph node metastases | |
| • N1 | |
| Metastasis in intrapulmonary and homolateral hilar and/or homolateral peribronchial lymph nodes | |
| • N2 | |
| Metastasis in the subcarinal and/or homolateral mediastinal lymph nodes | |
| • N3 | |
| Metastasis in supraclavicular, contralateral or homolateral scalene, contralateral hilar, or contralateral mediastinal lymph nodes | |
| M (distant metastasis) | |
| • MX | |
| The distant metastases cannot be evaluated | |
| • M0 | |
| There are no distant metastases | |
| • M1 | |
| There are distant metastases | |
| • M1a | |
| Existence of tumor nodule(s) separated from the primary tumor, in a lobe of the contralateral lung; tumor with pleural nodules or malignant pleural (or pericardial) effusionb | |
| • M1b | |
| There are distant metastases |
| 2. Stages | |||
| Hidden carcinoma | TX | N0 | M0 |
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 a,b | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T1 a,b | N1 | M0 |
| T2a | N1 | M0 | |
| T2b | N0 | M0 | |
| Stage IIB | T2b | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T1,T2 | N2 | M0 |
| T3 | N1,N2 | M0 | |
| T4 | N0,N1 | M0 | |
| Stage IIIB | T4 | N2 | M0 |
| Any T | N3 | M0 | |
| Stage IV | Any T | Any N | M1a,b |
Note: The changes from the previous TNM classification are in italics.
The very uncommon superficial dissemination of a tumor of any size with its invasive component limited to the bronchial wall, which may extend proximally until the main bronchus, is also classified as T1.
The majority of pleural (and pericardial) effusions associated with lung cancer are due to the tumor. However, there are some patients in whom multiple cytopathologic studies of the pleural (or pericardial) liquid are negative for tumors, the liquids is not bloody and is not an exudate. When these elements and the clinical judgment indicate that the effusion is not related with the tumor, the effusion should be excluded as a staging element and the patient should be classified as T1, T2, T3 or T4.
As was expected, the decrease in survival as the lymph node affectation increased was confirmed, and significant differences were found in the 5-year survival rate in three large groups of patients: (a) with affectation of only one pathological N1 area (48%); (b) multiple pathological N1 areas (35%) or only one pathological N2 (34%); and (c) multiple pathological N2 areas (20%). As these findings could not be validated by geographical areas or T categories, changes are not recommended regarding the N component for the new classification.4
It should be kept in mind that these data came from surgical patients whose lymphadenopathic state was evidenced by systematic lymph node dissection, which, for the moment, in clinical staging is only possible with video-assisted mediastinoscopic lymphadenectomy (VAMLA) or transcervical extended mediastinal lymphadenectomy (TEMLA) (see ahead, Procedures for staging with surgical techniques).
M ComponentThe patients studied presented the following survival rates at 1 and 5 years: T4 any N M0, 53% and 16%; pleural dissemination, 45% and 6%; contralateral pulmonary nodule(s), 46% and 3%, and distant metastasis, 22% and 1%; in this latter case, with significantly lower survival rates than previously cited.5 With such references, it was decided to subdivide the M component into M1a (presence of pleural dissemination or contralateral pulmonary nodule(s)) and M1b (distant metastasis) (Table 1).
Stage GroupingKnowing the previous arguments for reorganizing some sections of the T and M components, a sophisticated statistical study was carried out with 17,726 patients whose tumors were better staged.2 The different survival curves for each stage were obtained, which, without overlapping among them, presented worse levels as the tumor extension increased. This confirms the new stage grouping (Table 1), whose 5-year survivals for each stage were, according to clinical and pathological staging, respectively, the following: IA, 50% and 73%; IB, 43% and 58%; IIA, 36% and 46%; IIB, 25% and 36%; IIIA, 19% and 24%; IIIB, 7% and 9%, and IV, 2% and 13%.
Small-cell Lung Cancer (SCLC) and Carcinoid TumorsThe International Staging Committee of the IASLC has confirmed that the survival of patients with SCLC worsened as the T and N categories increased.6 It was also observed that, except in stage IIA, which had only 55 patients for analysis, the 5-year survival worsened as the stage progressed: IA, 38%; IB, 21%; IIA, 38%; IIB, 18%; IIIA, 13%; IIIB, 9%, and IV, 1%. Based on this, the proposal to use the TNM system for staging SCLC was confirmed.
Even though the 6th TNM classification specified that it was not applicable to carcinoid tumors, several studies have used it, finding prognostic differences among the stages. The IASLC has also confirmed that those classified as stage I lived significantly more than those in stage II, and these significantly more than those in stages III–IV; therefore, the new TNM classification of 2009 is recommended to describe the extension of these tumors.7
Limitations of the 2009 TNM ClassificationThe main limitations are derived from the retrospective character of some databases that were not designed to study the TNM classification and lack precise anatomical details about the tumor extension, the number and lymph node stations affected or the differences between the different forms of M1 disease. For this reason, the IASLC itself has initiated a prospective project aimed at once again updating the TNM classification in 2016, validating all the T, N and M descriptors, especially those who have not been until now. Thus, a large international database is being constituted that, correcting the geographical omissions and disproportions in the therapeutic modalities, includes patients with non-small-cell tumors, small-cell tumors and their neuroendocrine subtypes.
New Lymph Node MapThe IASLC has proposed a new lymph node map8 that has received international and multidisciplinary consensus which reconciles the differences between the map of Naruke and that of the Japan Lung Cancer Society with the map of Mountain and Dresler. The IASLC map maintains the lymph node stations of the other maps, but it also groups those that are anatomically proximal in lymph node areas in order to make the lymph node classification easier, especially in patients who will not undergo surgery. In this map, all the lymph node stations are defined by anatomically precise limits that are easy to recognize with imaging techniques and inspection during invasive explorations or thoracotomy. The innovations of this lymph node map are:
- •
The creation of a supraclavicular lymph node area that includes the supraclavicular, lower cervical (caudal on the lower edge of the cricoid cartilage) and the suprasternal fossa lymph nodes. If these lymph nodes are invaded by a tumor, they are classified as N3, regardless of the side of the tumor.
- •
The widening of the subcarinal lymph node station. It now includes all the lymph nodes from the tracheal bifurcation until the upper edge of the lower left lobar bronchus and the lower edge of the intermediary bronchus. If they are affected by tumors, these lymph nodes are classified as N2. This new subcarinal station includes lymph nodes that before, at least according to the Japanese map, were hilar (adjacent to the lower sides of the main bronchi), that could be classified as N1 or N3, depending on the side of the tumor. The larger size of this subcarinal station will mean an increase in N2 tumors in detriment of N1 and N3 tumors.
- •
The incorporation of precise limits for station number 10, the hilar station, which facilitates the prospective collection of data in order to clarify the prognostic role of this station, whose placement on other maps has always been controversial.
- •
The shift in the midline of the upper mediastinum from the tracheal anatomical midline to the left paratracheal margin exclusively affects the upper and lower right and left paratracheal stations. This modification implies that the affected lymph nodes that are to the left of the anatomical midline, but to the right of the new left paratracheal line, will be N2 for tumors of the right lung, but N3 for those of the left lung.
Without going into great detail, it is useful to remember their usefulness in staging. Detailed anamnesis and physical examination can provide significant data about the degree of extension of the disease, which would allow for a substantial simplification of the tests to be done later. Thus, dysphonia, superior vena cava syndrome, Horner's syndrome or thoracic pain often reflects the invasion of anatomical structures adjacent to the lung which usually contraindicate surgical treatment. Equally, neurological symptoms or intense, persistent bone pain should lead us to suspect the existence of distant metastasis, has relevant therapeutic and prognostic implications. Chest radiography is usually the first test to give a high-probability suspicion of the existence of LC. In addition to its diagnostic value, the detection of pleural effusion, destruction of vertebra or ribs, mediastinal invasion, etc., can be decisive for establishing the degree of extension and notably simplify the process of staging.
Thoracic Computed Tomography (CT)After chest radiography, CT is usually the next imaging test to provide relevant information in the staging process. Its performance and limitations in the diagnosis of LC have been extensively studied and are well-known9,10 (Table 2). Regarding the primary tumor (T), CT is still the best method for the overall anatomical study of the thorax. It obtains detailed information about the size, location, and anatomical relationships with neighboring structures of tumors and can detect very small nodules that would normally not be detectable with chest radiography. As for the invasion of the chest wall, there is a reported sensitivity (SEN) of 83% and specificity (SPC) of 80%, but the only truly reliable sign of invasion is bone destruction.9 With regards to the invasion of the mediastinum by the tumor, some criteria predict resectability with a distance between the mass and the mediastinum ≤3cm, the visualization of a fatty layer between the structures, or a contact angle between the mass and the aorta of less than 90°. Contrarily, the radiological signs suggesting invasion of the mediastinal structures that would implicate unresectability are not very reliable and it is not acceptable to rule out surgery based on such findings.9
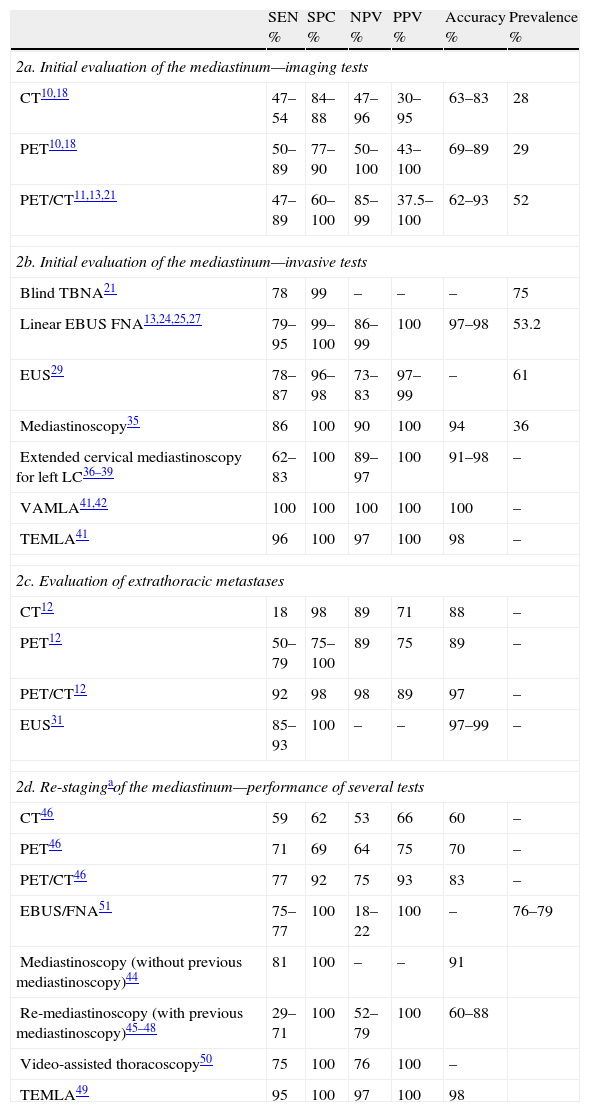
Diagnostic Performance of Several Tests in the Staging of LC.
| SEN % | SPC % | NPV % | PPV % | Accuracy % | Prevalence % | |
| 2a. Initial evaluation of the mediastinum—imaging tests | ||||||
| CT10,18 | 47–54 | 84–88 | 47–96 | 30–95 | 63–83 | 28 |
| PET10,18 | 50–89 | 77–90 | 50–100 | 43–100 | 69–89 | 29 |
| PET/CT11,13,21 | 47–89 | 60–100 | 85–99 | 37.5–100 | 62–93 | 52 |
| 2b. Initial evaluation of the mediastinum—invasive tests | ||||||
| Blind TBNA21 | 78 | 99 | – | – | – | 75 |
| Linear EBUS FNA13,24,25,27 | 79–95 | 99–100 | 86–99 | 100 | 97–98 | 53.2 |
| EUS29 | 78–87 | 96–98 | 73–83 | 97–99 | – | 61 |
| Mediastinoscopy35 | 86 | 100 | 90 | 100 | 94 | 36 |
| Extended cervical mediastinoscopy for left LC36–39 | 62–83 | 100 | 89–97 | 100 | 91–98 | – |
| VAMLA41,42 | 100 | 100 | 100 | 100 | 100 | – |
| TEMLA41 | 96 | 100 | 97 | 100 | 98 | – |
| 2c. Evaluation of extrathoracic metastases | ||||||
| CT12 | 18 | 98 | 89 | 71 | 88 | – |
| PET12 | 50–79 | 75–100 | 89 | 75 | 89 | – |
| PET/CT12 | 92 | 98 | 98 | 89 | 97 | – |
| EUS31 | 85–93 | 100 | – | – | 97–99 | – |
| 2d. Re-stagingaof the mediastinum—performance of several tests | ||||||
| CT46 | 59 | 62 | 53 | 66 | 60 | – |
| PET46 | 71 | 69 | 64 | 75 | 70 | – |
| PET/CT46 | 77 | 92 | 75 | 93 | 83 | – |
| EBUS/FNA51 | 75–77 | 100 | 18–22 | 100 | – | 76–79 |
| Mediastinoscopy (without previous mediastinoscopy)44 | 81 | 100 | – | – | 91 | |
| Re-mediastinoscopy (with previous mediastinoscopy)45–48 | 29–71 | 100 | 52–79 | 100 | 60–88 | |
| Video-assisted thoracoscopy50 | 75 | 100 | 76 | 100 | – | |
| TEMLA49 | 95 | 100 | 97 | 100 | 98 | |
CT: computed tomography; PET: positron emission tomography; TBNA: transbronchial needle aspiration; EBUS FNA: endobronquial ultrasound-guided fine needle aspiration; EUS: esophageal ultrasound; VAMLA: video-assisted mediastinoscopic lymphadenectomy; TEMLA: transcervical extended mediastinal lymphadenectomy.
In general, a diameter of 1cm at the shortest point is accepted as the upper limit of normal, although this criterion is not useful to discern between malignant and benign lymph nodes.9,10 Around 40% of mediastinal lymph nodes suggestive of malignancy according to CT are benign, and 20% of those that are apparently benign are not in the end. Even among patients in clinical stage 1A, 5%–15% will show lymph node affectation in the surgical-pathological examination.10 These limitations in diagnostic performance (Table 2) mean that the CT findings need to be confirmed with other more reliable tests.
Distant Metastasis (M)Liver and suprarenal glands. Isolated hepatic metastases are not frequent in NSCLC, but are in SCLC. The suprarenal glands are a frequent location for metastases, although their differentiation with benign adenomas often requires obtaining cytohistological samples. Therefore, during the same exploration, both chest and upper abdominal CTs are usually performed. As for the search for possible extrathoracic metastases, the recommendations are commented further ahead.
Positron Emission Tomography (PET and PET/CT)PET, a diagnostic modality based on the greater metabolic activity of the neoplastic cells, provides information of interest about tumor biology, but its capacity for spatial resolution is less than that of CT. As for the threshold of normality for the so-called standard uptake value (SUV), each center should establish its own cut-point. The development of PET/CT, which integrates the images of both procedures into one single exploration, improves diagnostic accuracy.11,12 For the evaluation of the mediastinum, said efficacy is higher than that of CT (Table 2), although it varies depending on the type: thus, for adenocarcinoma, the PPV of PET/CT is 50%, and the NPV is 77.8%; meanwhile, for squamous carcinoma said levels are 23.1% and 96.3%, respectively.13
Nevertheless, the high captation of glucose in benign processes such as granulomas and infections causes a rate of false positive results (FP) of 20%–25%. It is therefore recommended to confirm such findings by obtaining cytohistological samples before rejecting the option of surgery in a patient who is a potential candidate. Contrarily, given a negative PET result in the mediastinal evaluation, it is considered acceptable to proceed with the intervention without previous invasive tests, with the following exceptions: (a) centrally located tumors, usually in contact with the mediastinum; (b) tumors with low metabolic activity; (c) apparent N1 affectation; or (d) when CT detects lymph nodes with tumors whose smallest diameter is >15mm; in this latter situation, a meta-analysis revealed a post-test probability for tumor affectation of 21%.14,15
The PET or PET/CT results, given their high SEN for detecting distance metastasis, can be relevant for modifying the therapeutic plan, especially to avoid unnecessary thoracotomies.16 On the other hand, in patients who are candidates for radiotherapy, PET/CT can better outline the area to be radiated.17 Thus, its use is recommended for patients with clinical stages 1A–IIIA, provisionally subsidiaries for radical treatment, even though its usefulness in stage IA is less evident.10
Search for Extrathoracic MetastasesA careful clinical evaluation is still the best method for the prediction of metastases. Non-specific symptoms, such as weight loss, asthenia, and osteomuscular pain or other more specific symptoms, such as subtle changes in mood or mild loss of strength in a limb, as well as biochemical or hematological alterations that are unexplained by any other reason (hypercalcemia, hypoalbuminemia, high LDH, anemia, etc.), are associated with the presence of metastasis. The diagnostic imaging tests are guided by the location of the present symptoms or signs. Thus bone pain justifies performing a gammagraphy, which has a SEN of 87% and an SPC of 67% for detecting bone metastasis.10 Given the frequent existence of degenerative or posttraumatic lesions, it is not rare to find doubtful images, in which case PET can be useful, as it is highly exact with SEN, SPC, PPV and NPV above 90%.9 PET, or PET/CT, are more resolute than CT for discerning hepatic or suprarenal lesions, especially when they are large, in which case the exactness is close to 100%. In lesions with a diameter of <15mm, the performance is much lower. In addition to its greater diagnostic efficacy in the usual locations, PET can detect metastases or other primary tumors in unsuspected places, such as the gastrointestinal tract, bladder, soft tissue, etc. Recent publications demonstrate that PET/CT could improve the performance of isolated PET or CT, although 15% of the abnormal images are classified as indeterminate.12,18
Table 2 summarizes some results from said studies, although the levels of NPV could be overestimated due to limitations in the follow-up derived from the short time that has passed since the introduction of PET/CT. The systematic practice of this test improves staging by detecting distance metastases left hidden after conventional staging (from 8% in patients in provisional stage I until 24% in stage III, using PET/CT), reducing in this manner the number of useless thoracotomies.16
But it is not free of disadvantages, as the existence of false positive (FP) results often requires carrying out risky procedures in order to obtain cytohistological confirmation of a lesion that finally turns out to be benign. In general, except for overwhelming clinical and radiographic evidence, the findings of images suggestive of metastasis, especially when it is a single, focal hypercaptation, should not lead to the exclusion of the patients from potentially curative treatments if histological confirmation of malignancy is not obtained.18 On the other hand, it must be remembered that, in some cases, the confirmation of a single metastasis (suprarenal or cerebral) does not necessarily imply renouncing surgical treatment of the primary tumor, as it could also be removed.8
With regards to the diagnosis of cerebral metastases, cranial CT or magnetic resonance imaging (MRI) should be carried out in cases with any suspicious neurological symptoms or signs, as well as in asymptomatic stage III patients in whom the possibility of aggressive treatment (surgery or thoracic radiotherapy) is considered.19 In earlier stages, although the indication is more arguable, given the greater incidence in the non-squamous types (even up to 20%), it seems recommendable.
Thoracic MRIIts use is recommended only under special circumstances, such as in tumors of the sulcus superior due to its superiority over CT for evaluating the invasion of the brachial plexus, the mediastinal vessels or the vertebral body. In such cases the diagnostic exactness for evaluating the tumor extension can reach 94%, compared to 63% with CT.9
Procedures for Non-surgical Invasive StagingEndoscopic explorations, both digestive and respiratory, are used for obtaining cytohistological samples from the mediastinal lymph nodes using fine-needle aspiration (FNA). Transbronchial aspirations can be done with two methods: blind (transbronchial needle aspiration [TBNA]) or real-time ultrasound-guided (endobronchial ultrasound [EBUS]). Transbronchial aspiration can access the high mediastinal (2, 3p and 4), subcarinal (7) and the intrapulmonary hilar (10) and lobar (11) stations. Transesophageal TBNA (EUS-TBNA) is done with ultrasound control in real time and can reach the lower left paratracheal (4L), frequently the subaortic (5) and all the lower mediastinal (7, 8 and 9) stations. On some occasions, if the adenopathy is located relatively posterior, it is possible to also access 4R, 2R and 2L. EBUS and EUS-TBNA of mediastinal lesions are safe procedures that can be done on an outpatient basis. No important complications have been described in the needle aspiration of mediastinal adenopathies.20
Although there is no consensus about the standards for endoscopic ultrasound explorations, it would be recommendable to: (1) explore and aspirate all the suspicious lymph nodes seen on PET-CT, sequentially ruling out N3, N2 and N1; and (2) explore the N3 lymph node stations in all the cases with the intention for radical curative treatment, and aspirate the lymph nodes ≥5mm in diameter. Recent studies indicate that we should consider the result of the endoscopic ultrasound negative when no evidence for malignancy is found after 3 aspirations with the presence of lymphocytes in the perioperative cytological examination. Likewise, if the sample is contaminated, necrotic, insufficient or bloody, it should be considered indeterminate, and the negativity for malignant cells should be confirmed by means of surgical techniques.
Blind Transbronchial Needle Aspiration (TBNA)In a systematic review of 17 studies, it has been established that TBNA has a SEN of 78%, a SPC of 100% and an FN rate of 28%.21 The diagnostic accuracy only comes close to ultrasound-guided needle aspiration in the subcarinal station; in the rest, its performance is poorer (58% vs 84%).22 When cost-efficient criteria are introduced, TBNA is less recommendable than endoscopic ultrasound techniques and mediastinoscopy (MED) when the prevalence of mediastinal lymph node affectation is higher than 25%.23
Endobronchial Ultrasound (EBUS)In a meta-analysis centered on the staging of LC by EBUS, a total of 11 studies were reviewed, including 1299 patients.24 The total SEN was 93% and the total SPC was 100%, with a mean prevalence of 53.2%. The analysis by subgroups shows that the selection of patients with abnormal lymph nodes on CT or PET and the availability of immediate cytopathologic diagnosis are independent factors that increase the total SEN to 94% and 97%, respectively. In the absence of immediate cytohistological diagnosis, the diagnostic performance depends on the number of needle aspirations done per lymph node. SEN is 69.8%, 83.7%, and 95.3% for the first, second and third aspirations, while the NPV is 86.5, 92.2, and 97.6%, respectively.25
The high diagnostic precision of EBUS for mediastinal lymph node staging even holds true for lymph nodes with a diameter <1cm. In a study of 100 patients with normal lymph nodes on CT (diameter between 5 and 10mm; mean, 8.1mm) a SEN was obtained of 92.3% and a SPC of 100%, with a NPV of 96.3%.26 In all, surgical controls were carried out using thoracotomy (85%) or MED (15%). And in a second study with 100 patients with LC and PET with a morpho-metabolically normal mediastinum, EBUS-TBNA had a SEN of 89%, a SPC of 100% and a NPV of 98.9% in the detection of lymph node metastases.27
Data have not been published for the cost-efficiency analysis, although it can save between 30% and 56% of mediastinoscopies.28
Endoscopic Ultrasound (EUS)In a meta-analysis grouping 18 studies, it was concluded that the total SEN of the technique is 83% and total SPC 97%. When the examination is done in patients with pathologic CT, the SEN increases to 90%, with a SPC of 97%.29 It is possible to aspirate lymph nodes in patients with normal mediastinum on CT (diameter<1cm), with a SEN between 50% and 61% and a SPC between 98% and 100%.30
EUS-TBNA can also detect sub-diaphragmatic metastases (left suprarenal gland, lymph nodes of the celiac trunk and liver)31 and evaluate the presence of mediastinal invasion by the tumor (T4) with much more precision than with radiological techniques (SEN, 98%; SPC, 98%; FN, 1%, and FP, 30%).32
In a comparative randomized study, between MED and systematic EUS-TBNA in 104 patients candidates for surgery, it was demonstrated that the latter alternative reduced the number of unnecessary thoracotomies from 25% to 9%.33
Global Mediastinal Ultrasound ExplorationThe combination of EUS-TBNA and EBUS-TBNA enables complementary access to all the mediastinal lymph node stations, except the 6th. The SEN of this combination is 93%, with a NPV of 97%. In a cost analysis model from the year 2007 and according to the standards of American health insurance providers, it was estimated that the combination of EBUS and EUS is more cost-efficient than MED with the prevalence of affected lymph nodes over 32.9%. Under this percentage, the most cost-efficient approach is EUS.23
Procedures for Staging With Surgical TechniquesMediastinoscopy (MED), Mediastinotomy, Extended Cervical Mediastinoscopy (ECM) and Video-assisted ThoracoscopyThe two most recent guidelines for clinical practice13,21 about the clinical staging of LC coincide in recommending, with a degree of recommendation 1B,21 the cytohistological confirmation of the radiological or metabolic alterations that suggest mediastinal lymph node affectation. Both guidelines, completed before recent publications about the performance of EBUS27 and EUS,29 also recommend a minimally invasive surgical technique if this confirmation was done with an endoscopic technique and the results of the cytohistological study of the sample were either negative for malignancy or inconclusive.
In these cases, the most widely used surgical technique is MED. It can explore the right and left, upper and lower paratracheal lymph node stations as well as the subcarinal station. The minimal acceptable requirement for MED is the biopsy of at least one lymph node of the lower right and left paratracheal and the subcarinal stations.13 If the tumor is on the left, it is necessary to extend the exploration to the subaortic and para-aortic lymph node stations, especially if the tumor is hilar or of the upper lobe. Both the left parasternal mediastinotomy and the ECM give access to these two lymph node stations. The experience with 739 systematic mediastinotomies for staging left LC demonstrated the increase in positive explorations when combined with MED (28% of the explorations). In addition, the systematic sampling of the mediastinal fat when no lymphadenopathies are found at these levels reveals neoplastic infiltration in 6% of the cases.34 Systematically carried out in combination in patients who are candidates for lung resection, left parasternal MED and mediastinotomy or ECM reach the following results: SEN 86%; SPC 100%; diagnostic accuracy 94%; PPV 100%, and NPV 90%.35 When these explorations are negative, the possibility for finding metastasized lymph nodes in the lower mediastinal lymph node stations is very low (1.2% of cases)35; therefore its systematic exploration with thoracoscopic or ultrasound-guided transesophageal needle aspiration, when the imaging techniques are normal, would not be indicated. The rate of complications of these explorations is around 3%, and the mortality rate is around 0.1%.
There are few publications about ECM as a substitution for left parasternal mediastinotomy for staging left LC, but the results are very homogeneos.36–39 It is a valid alternative to left parasternal mediastinotomy with no specific associated complications. Its high NPV is relevant for indicating lung resection without the need for induction treatment.
Thoracoscopy, with or without video-assistance, provides a complete exploration of the pleural cavity and the ipsilateral mediastinum, if there are no pleural adherences that impede it. Under ideal conditions, it gives access to the lower right paratracheal, hilar, subcarinal and paraesophageal lymph node stations and the lower lung ligament on both sides, as well as the subaortic and para-aortic stations on the left side. Compared with MED, it has the disadvantage of being a unilateral exploration, and therefore it cannot rule out N3 affectation, except when both hemithoraces are explored. It is useful in the study of accompanying pleural effusions in order to confirm or rule out pleural affectation, and in the diagnosis of lung nodules if their peripheral location allows them to be biopsied or removed by means of atypical lung resection. Its use before thoracotomy, associated with pericardioscopy, can identify causes of unresectability and avoid exploration thoracotomies in 10% of patients.40
The adaptation of a video camera to the mediastinoscope has made the technique evolve in an unexpected way. Two methods of mediastinal lymphadenectomy by video-assisted MED have been described, which are equivalent to mediastinal lymph node dissection performed by thoracotomy. These are video-assisted mediastinoscopic lymphadenectomy (VAMLA)41,42 and transcervical extended mediastinal lymphadenectomy (TEMLA)43 (Table 2). It is interesting to note that, in the last 150 TEMLA, all the diagnostic parameters were 100%.
All these techniques are also useful for re-staging and are indicated in the pre-operative evaluation of second primary tumors, recidivated tumors and, currently more frequently, in the evaluation of tumor response after induction treatment in cases of locally advanced LC. For this latter indication, Table 2 summarizes the results of MED for re-staging without previous MED,44 re-mediastinoscopy (REMED),45–48 TEMLA49 and video-assisted thoracoscopy.50 In the case of MED for re-staging without previous MED, it is logical that the levels are higher than those of REMED, as the upper mediastinum is not altered by the adherences of the first surgical procedure. The results of REMED are very homogeneous when done systematically. Only one series stands out due to its low SEN and low NPV. However, in that series, in more than 60% of patients, in REMED the subcarinal lymph nodes were not biopsied, which are the most affected lymph nodes together with the lower right paratracheal nodes. TEMLA has the best values for re-staging, but it must be kept in mind that, in these cases, the staging was done by means of EBUS. Therefore, there were no mediastinal adherences, which are what complicate REMED. Last of all, video-assisted thoracoscopy has been used very little in re-staging, but the results of this unique multi-centered study50 indicate that they may be comparable to those of REMED.
Re-stagingAlthough the combination of chemotherapy and thoracic radiotherapy (TRT) is the treatment indicated in the majority of patients with mediastinal tumor affectation, N2, some with favorable circumstances for foreseeable lobectomy can benefit from surgical resection, as long as the induction treatment achieves complete histological remission of the mediastinal lymphadenopathies. The persistence of tumors in said lymphadenopathies is generally considered a contraindication for surgery. From here stems the crucial importance of a precise evaluation of the lymph node response to induction treatment. CT can detect changes in tumor size that take place after induction treatment, but even in cases of large reductions in the post-chemotherapy tumor volume, clones of viable malignant cells often persist. On the other hand, it is also known that residual masses of considerable volume can be constituted by fibrotic tissue alone. In this regard, PET and PET/CT have recently demonstrated greater preciseness in the evaluation of the response to treatment, although the diagnostic performance is substantially less than in initial staging (Table 2). Thus, it is essential to obtain a cytohistological sample from the mediastinal lymph nodes after induction treatment. EBUS has a SEN of 76% and a SPC of 100% for analyzing the response to induction chemotherapy. However, its NPV is 20%, which requires surgical confirmation.51 Stigt et al., in a group of 26 patients who received induction chemotherapy and confirmation by EUS, obtained a NPV of 91.6%.52 To date, there are no studies on combined endoscopic re-staging.
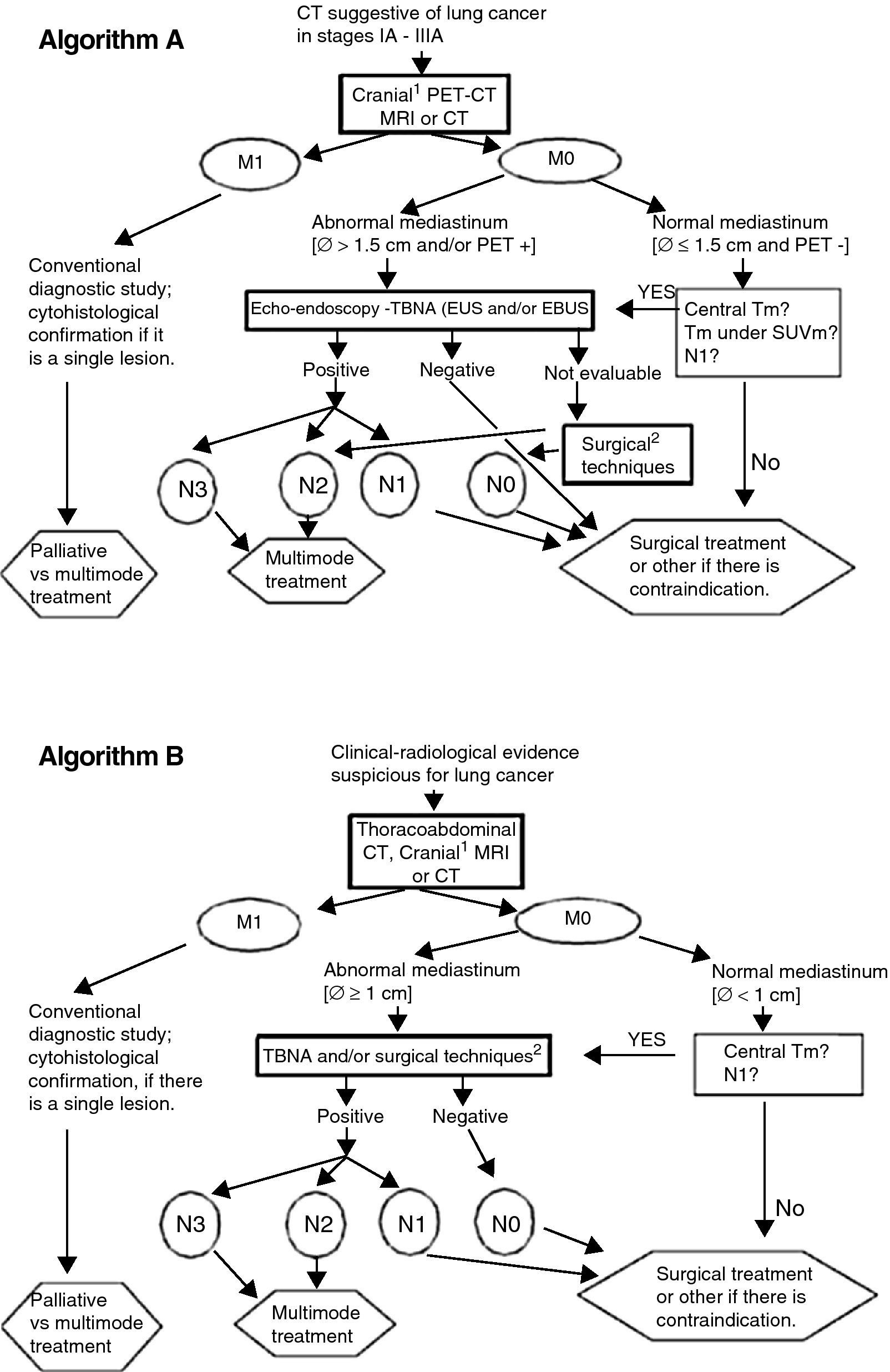
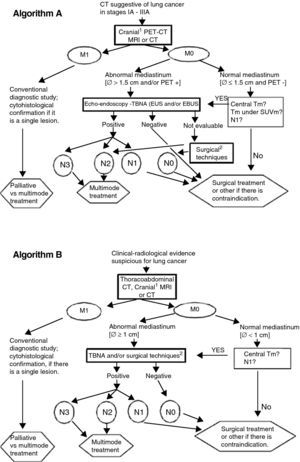
After evaluating the recent information about the performance of several tests, Fig. 1 recommends lines of action adapted to the available means. Algorithm B, although not optimal, may still be the only option available in some centers.
Lung cancer staging algorithms. (A) Cranial MRI for patients who are candidates for radical treatment in stage III. (B) Surgical techniques: mediastinoscopy, mediastinotomy, extended cervical mediastinoscopy, thoracoscopy, transcervical extended mediastinal lymphadenectomy (TEMLA) and video-assisted mediastinoscopic lymphadenectomy (VAMLA). Notes: (a) EBUS results will be considered negative only if, after at least three needle aspirations with presence of lymphocytes, no malignant cells are observed; (b) algorithm B may be acceptable in centers without accessibility to PET, EBUS or EUS; (c) The limit of 1.5cm for the size of the mediastinal lymph nodes in algorithm A is based on a meta-analysis (see text and Ref. 14). The 1-cm limit (algorithm B) is traditionally used.
Correct tumor and lymph node pathological classification is very important to provide a prognosis, indicate adjuvant treatment and carry out comparisons in clinical studies.
Surgical Pathological StagingEvaluation of T Tumor AffectationDuring surgery, histological samples should be taken from the anatomical structures that are suspicious macroscopically of tumor invasion and that modify the T classification of the tumor. The study will be intraoperative and, if positive, the extension of the necessary resection may vary. Although these situations should be evaluated pre-operatively, in certain T4 affectations complete resection of the tumor, from the tracheal carina, laryngeal nerve or vertebral body, can be achieved.
In these cases, the N2 affectation should also be excluded intraoperatorily.53 Patients with a satellite nodule in the same lobule of a diagnosed LC do not require intraoperative investigation.54 Finally, if during surgery another tumor is found in a different lobe, both tumors should be treated once N2 affectation is also excluded intraoperatively.54
Evaluation of the N Lymph Node AffectationThe best way to classify lung resection is intraoperative lymph node staging. The impact of lymph node affectation in the prognosis makes it easier to make decisions about the indication of adjuvant treatments. It is considered a key element to consider surgery and it is an essential requirement in surgical quality control.55 The techniques used go from simple inspection to en bloc bilateral lymphadenectomy. The extension of the lymph node evaluation will depend on the prognostic implications, the possible inclusion in complementary treatments, even on the possibility of local relapse of the disease. The balance between the risk of an exhaustive and extensive exploration with the associated morbidity and the correct classification of the lymph node affectation should guide any and all decision-making.
The clinical assay of the American College of Surgery Oncology Group (ACOSOG Z0030)54 designed for determining the effect of lymphadenectomy on survival in patients operated on due to LC did not demonstrate differences in the morbidity and mortality between lymphadenectomy and lymph node sampling. Although the results of this study on survival have still yet to be communicated, a meta-analysis, carried out by Wright et al.56 with 3 clinical assays with few patients and important methodological limitations, suggests that lymphadenectomy improves survival compared with lymph node sampling.
Although in multi-station affectation, both N1 and N2, seem to have a worse prognosis than just any one alone,4 there is no evidence for modifying the evaluation recommendations.
The intraoperative evaluation guidelines of the ESTS57 propose the following definitions about lymph node evaluation methods during surgery:
- 1.
Selective lymph node biopsy. This consists of biopsying one or several suspicious-looking lymph nodes. This would be justified only for demonstrating the N1 or N2 affectation when resection is not possible (explorational thoracotomy).
- 2.
Sampling. This is the exploration with extirpation of lymph nodes from a pre-established number of pulmonary and mediastinal lymph node stations with a specific objective. Systematic sampling: resection of pre-determined stations by the surgeon.
- 3.
Systematic lymph node dissection. Systematic resection of all the mediastinal tissue, including the lymphadenopathies between the anatomical limits. In the tumors of the left side, in order to be able to access the upper and lower paratracheal stations, the arterial ligament should be freed in order to be able to move the aortic arch. A minimum of 3 lymph node stations are required (always including the subcarinal). The lymph nodes should be identified and analyzed histologically separately. In addition, the hilar and intrapulmonary lymphadenopathies should be dissected.
- 4.
Lobe-specific systematic lymph-node dissection. Systematic resection of all the mediastinal tissue, including lymphadenopathies, depending on the lobe where the tumor is located. This type of selective dissection is applicable to T1 squamous carcinomas. The areas to be explored, depending on the lobe affected and according to the descriptions by Naruke58 and Ichinose59 and the guidelines of the Bronchogenic Carcinoma Cooperative Group of SEPAR,55 are: dissection and histological examination of the hilar and intrapulmonary (lobar, interlobar and segmental) lymph nodes and at least three of the following mediastinal lymph node stations, according to the lobar location of the primary tumor: (a) right upper lobe and middle lobe: areas 2R, 4R and 7; (b) right lower lobe: areas 4R, 7–9; (c) left upper lobe: areas 5–7; (d) left lower lobe: areas 7–9. In total, 6 lymph nodes should be included.
- 5.
Extended lymph node dissection. This is defined as the dissection of the contralateral mediastinal and pulmonary lymph nodes, complementary to the systematic dissection of the lymph nodes ipsilateral to the tumor. It includes cervical dissection and is generally performed by means of mid-sternotomy.
For patients with tumors in stage I or II who can tolerate lung resection, the minimum procedure recommended is lobectomy.60 For patients with stage I who can tolerate a surgical intervention but not a lobectomy, sublobar resection (regulated or atypical segmentectomy) is preferable to non-surgical treatment.60 In stage I, the approach by means of minimally invasive surgery is as recommendable as standard thoracotomy.61 In patients with central or locally advanced tumors, as well as in those with N1, complete resection by means of bronchoplastic techniques offers better results than pneumonectomy.62
Definition of complete tumor resection. The IASLC defines complete tumor resection63 as that which meets the following criteria:
- (a)
Free resection margins, as demonstrated microscopically. These margins should include the stumps of arteries, veins and bronchi, peribronchial soft tissue and any peripheral margin close to the tumor or to the rest of the resected tissue.
- (b)
Systematic lymph node dissection as extensive as possible, or rather lobe-specific systematic lymph node dissection (as previously described). The samples should include at least six lymph nodes: three of the intrapulmonary and/or hilar stations and three of the mediastinal stations, one of which should be subcarinal.
- (c)
There should not be isolated extracapsular extension of the tumor in the resected lymph nodes or in those located in the margin of the main lung tumor.
- (d)
The highest removed mediastinal lymph node should be negative.
Processing and macroscopic study. The piece should optimally be set by inflating the airway with formaldehyde as a routine fixative. The measurements of the surgical piece and the tumor are taken once the piece is fixed.
The evaluation of the tumor should include: (a) parenchymatous or endobronquial location (if the bronchus is main, lobar or segmental) and distance from the pleura; (b) largest diameter; (c) description (shape, color, delimitation, cavitation); (d) anatomical extension: bronchial affectation, vascular invasion, visceral pleural invasion or the shortest distance from the visceral pleura (the IASLC recommends the use of staining for the elastic layer of the visceral pleura, whose affectation defines the invasion of the visceral pleura, in cases in which normal staining with hematoxylin and eosin are doubtful),64,65 extension to interlobar fissures and adhered tissues; (e) distance from margins, specifying the shortest distance (bronchial, vascular, surface of the resected parenchyma, adhered tissues); and (f) presence of other tumor nodules. Synchronic primary carcinomas are independently staged.
Evaluation of the surrounding parenchyma. Normal or abnormal, specifying the pathology.
Lymph nodes of the surgical piece. Location, number and size.
Lymph nodes remitted separately from the piece. With specifications of each lymph node station, number and size.
Molecular Staging of Lung CancerThe presence of lymph node metastasis is one of the most important prognostic factors in NSCLC. Between 30% and 40% of patients without apparent lymph node affectation at the time of the surgery present relapse of the tumor, causing death. This sub-staging probably results from the existence of hidden tumor cells that are impossible to detect by standard imaging or histopathological methods.
The term micrometastasis is accepted as being a focus of tumor cells between 0.2 and 2mm in diameter that are usually only detectable by means of immunohistochemistry. In LC, we look for proteins associated with epithelial cells, such as cytokeratins. Different studies concur that the presence of micrometastasis is irrefutably associated with a shorter disease-free period and poorer overall survival.
Molecular staging refers to the determination of tumor biomarkers in the lymphatic tissue as an indicator of the presence of neoplastic cells. The detection and quantification of messenger RNA that codifies proteins of tumors or epithelial cells is possible using quantitative reverse-transcriptase-polymerase chain reaction (qRT-PCR).
It is also possible to analyze tumor epigenetic alterations or specific mutations in the DNA extracted from lymph nodes. The need for a minimal quantity of tissue, a greater sensitivity for detection and lower costs are some of the advantages of the use of qRT-PCR over immunohistochemistry.
Several studies analyze the value of different biomarkers in samples of surgically removed lymph nodes.66,67 It is also possible to study the presence of biomarkers in mediastinal lymph nodes using endoscopic ultrasound needle aspiration techniques, although the studies published in this regard are limited.68,69 The presence of methylation or of certain mutations in the genes studied is more frequently related with tumor relapse and with lower total and disease-free survival. However, still there are neither large patient series with long follow-ups nor randomized studies that can affirm without a doubt that molecular staging has clinical and prognostic relevance. Nevertheless, identifying this group of patients in the near future will allow them to benefit from some type of chemotherapy or other drug therapy to reduce the risk of relapse.
Summary of Recommendations- 1.
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) accepts and recommends employing the 7th edition of the TNM classification proposed by the IASLC in 2009 and the new lymph node map of the IASLC for staging NSCLC, SCLC and carcinoid tumors.
- 2.
Chest and upper abdominal CT should be carried out for all patients with suspicion or diagnosis of LC which may be susceptible to treatment. Grade of recommendation (Gr. R): consistent; evidence (Ev): moderate.
- 3.
In patients with clinical stages IA–IIIA who are potential candidates for radical treatment, PET or PET/CT are indicated for the evaluation of the mediastinum and detection of possible extrathoracic metastases. Gr. R.: consistent; Ev: moderate (weak in stage IA). In the absence of M1, if PET shows hypermetabolism in the mediastinal lymph nodes, cytohistological confirmation is necessary (GrR: high/Ev: high). If the PET is negative, surgical treatment can proceed directly, except under the following circumstances: (a) mediastinal lymph nodes whose smallest diameter is >15mm on CT with contrast; (b) a central tumor (middle 1/3 of the hemithorax), usually in contact with the mediastinum; (c) the tumor has low SUVmax (like some adenocarcinomas); or (d) there is suspicion for N1. Under these circumstances, cytohistological confirmation of the mediastinal lymph nodes is recommended prior to surgery (GrR: consistent/Ev: moderate). The combination of EBUS and EUS is the endoscopic approach with the best diagnostic performance (GrR: consistent/Ev: high).
- 4.
A negative result of a needle aspiration should be confirmed by means of mediastinoscopy (GrR: weak; Ev: high) when three samples have not been taken in absence of immediate cytopathologic diagnosis, or rather if the cytopathologic diagnosis does not confirm presence of normal lymphatic tissue.
- 5.
Cranial CT or MRI are recommended when given any suspicious neurological symptom or sign and in neurologically asymptomatic patients with stage III in which the possibility of radical treatment is considered (surgery or thoracic radiotherapy). Gr. R.: consistent; Ev: moderate. In earlier stages and non-epidermoid types, it seems useful, although there is less evidence.
- 6.
The finding of an abnormal image suggestive of single metastasis should be confirmed with a cytohistological sample of the lesion before excluding the patients from potentially curative treatments. Gr. R.: consistent; Ev: moderate.
- 7.
When there is a foreseeable possibility for surgery after induction treatment (stages IIIA–N2), it is recommended to re-stage the mediastinal lesions by obtaining a cytohistological sample. If the procedure used has been a needle aspiration and the result is negative, a surgical technique for confirmation is recommended. Gr. R.: consistent; Ev: high.
- 8.
Among the different possible approaches (TBNA, EUS-TBNA, EBUS-TBNA, MED, mediastinotomy, VAMLA or TEMLA) for obtaining cytohistological samples, the technique should be chosen depending on experience, while the technique that is most cost-efficient, less invasive and has the least delay should prevail. Each center should plan the sequence of possible tests to be done depending on their local availability so that the start of treatment is delayed as little as possible. Gr. R.: consistent; Ev: weak.
- 9.
Systematic lymph node dissection is the recommended intraoperative lymph node evaluation because it ensures correct staging in order to indicate proper adjuvant treatment. It has no increased morbidity or mortality compared with lymph node sampling and it seems to be associated with a better prognosis. Gr. R.: consistent; Ev: high.
Please cite this article as: Sánchez de Cos J, et al. Normativa SEPAR sobre estadificación del cáncer de pulmón. Arch Bronconeumol. 2011;47:454–65.