Lymphangioleiomyomatosis (LAM) is a rare lung disease that predominantly affects young females and generally progresses to respiratory failure. There is not sufficient evidence to support the routine use of any treatment in LAM. The only treatment for severe LAM is currently lung transplantation. Activation of mammalian target of rapamycin (mTOR) signalling pathway has been observed in LAM. LAM is often associated with angiomyolipoma in the kidneys. mTOR inhibitor sirolimus reduces angymiolipoma volumes. Some reports have shown improvement in lung function with sirolimus in LAM.
We report 3 women with LAM, with a rapid decline in lung function and symptoms and who were treated with sirolimus.
La linfangioleiomiomatosis (LAM) es una enfermedad pulmonar rara que afecta a mujeres jóvenes y que suele progresar hacia el fracaso del sistema respiratorio. No existe evidencia científica suficiente que justifique el uso de ningún fármaco en la LAM. El único tratamiento efectivo en los casos graves es el trasplante pulmonar. En la LAM se ha observado una activación de mammalian target of rapamycin (mTOR). La administración de sirolimus, un inhibidor de mTOR, parece reducir los angiomiolipomas renales que se asocian a la LAM. Además, algunos trabajos sugieren una mejoría de la función pulmonar con este fármaco. Presentamos tres mujeres con LAM que manifestaron un deterioro clínico y funcional respiratorio rápidamente progresivo y que fueron tratadas con sirolimus.
Lymphangioleiomyomatosis (LAM) is a rare lung disease that affects young women of fertile age. It is characterized by the proliferation of atypical smooth muscle cells (“LAM calls”) in the lung interstitium and around the bronchovascular structures as well as by the formation of parenchymal cysts, which lead to a progressive loss of lung function.1 Two clinical forms are seen: a sporadic form and another associated with tuberous sclerosis (TS).
To date, there is no drug treatment that has been shown to modify the course of the disease. For years, progesterone has been the most widely used therapy in this disease, although there is not enough scientific evidence to recommend its routine use.
Patients with LAM in functional class III or IV of the New York Heart Association (NYHA) with hypoxemia at rest and with significant functional deterioration should be remitted to a lung transplantation reference center.
The latest advances in the fields of cell biology in LAM show the existence of somatic mutations in the genes of TS. Sirolimus, an mTOR inhibitor, could counteract the resulting abnormalities. A clinical trial in phase III with sirolimus is currently underway.
We describe the evolution of the lung function of three patients with LAM, two with sporadic LAM and one with LAM associated with TS, all of whom received treatment with the compassionate use of sirolimus.
Clinical ObservationsCase 1The patient is a 44-year-old woman, ex-smoker, with sporadic LAM diagnosed by surgical lung biopsy in 2007. There had been no pneumothorax or chylothorax, nor was there any presentation of renal angiomyolipomas on abdominal CT. The patient's symptoms included progressive dyspnea on exertion that had intensified over recent months. At the time of the diagnosis, the lung function tests showed: FEV1 1730ml (66.1%), FVC 3720ml (122%) and DLCO 3.35mmol/min/kPa (40.9%). Four months afterwards, a significant decline in lung function was observed: FEV1 1380ml (52%) and DLCO 2.37mmol/min/kPa (29%), although the FVC was 3870ml (127%). The 6-min walk test showed a distance walked of 486m, initial baseline oxygen saturation of 94% and ending at 87%. A decision was made for treatment with the compassionate use of oral sirolimus at 2mg/day. Serial analyses were done to control the levels of the drug. Thirty-five months after the start of treatment, lung function showed: FEV1 2100ml (82.7%), FVC 4810ml (161%) and DLCO 3.91mmol/min/kPa (48.7%) (Table 1). Likewise, the 6-min walk test showed improvement, with a total distance walked of 542m, and initial and ending O2 saturations of 95% and 90%, respectively. During follow-up, the only side effects presented were oral ulcers and headaches.
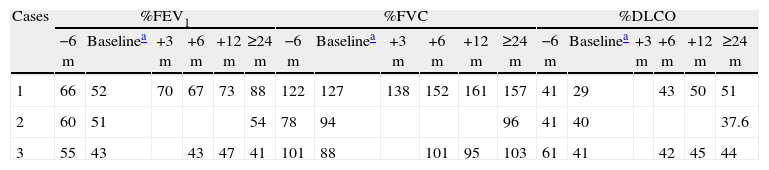
Evolution of the Lung Function.
| Cases | %FEV1 | %FVC | %DLCO | |||||||||||||||
| −6m | Baselinea | +3m | +6m | +12m | ≥24m | −6m | Baselinea | +3m | +6m | +12m | ≥24m | −6m | Baselinea | +3m | +6m | +12m | ≥24m | |
| 1 | 66 | 52 | 70 | 67 | 73 | 88 | 122 | 127 | 138 | 152 | 161 | 157 | 41 | 29 | 43 | 50 | 51 | |
| 2 | 60 | 51 | 54 | 78 | 94 | 96 | 41 | 40 | 37.6 | |||||||||
| 3 | 55 | 43 | 43 | 47 | 41 | 101 | 88 | 101 | 95 | 103 | 61 | 41 | 42 | 45 | 44 | |||
%DLCO: percentage of the predicted lung diffusion capacity; %FEV1: percentage of the predicted forced expiratory volume in 1s; %FVC: percentage of predicted forced vital capacity.
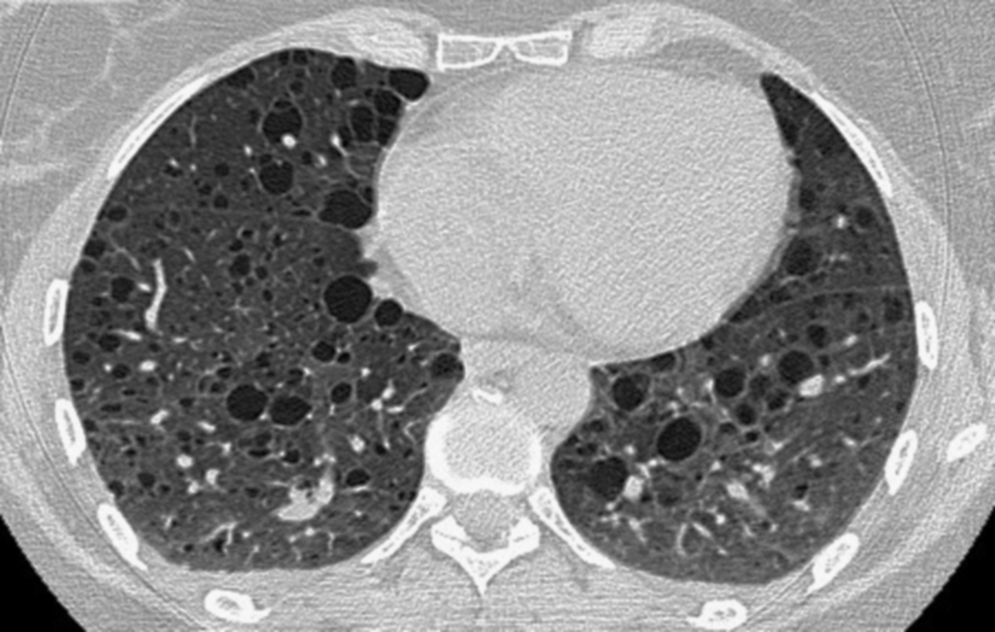
The patient is a 40-year-old woman, with no history of smoking, diagnosed with TS in 1982. In October 2006, the patient was seen for progressive dyspnea on exertion. Thoracic CT showed evidence of the presence of multiple thin-walled cystic lesions distributed diffusely in both lung fields (Fig. 1), establishing the diagnosis of LAM associated with TS. Bilateral renal angiomyolipomas were also observed. In October 2007, the patient had an episode of chylothorax that required the placement of an endothoracic drainage tube. Functional tests showed: FEV1 1680ml (60%), FVC 2540ml (78%) and DLCO 3.5mmol/min/kPa (41.1%); over a period of 8 months, there had been a reduction of 200ml in FEV1 as well as worsened dyspnea symptoms. With the patient's consent, it was decided to initiate treatment with oral sirolimus at a dosage of 2mg/day. During the 24-month follow-up, the symptoms and decline in lung function have stabilized. Current levels are: FEV1 1460ml (53.6%), FVC 3050ml (94%) and DLCO 3.15mmol/min/kPa (37.6%) (Table 1). The patient has shown no side effects from the medication, despite the dose having been increased to 3mg.
Case 3The patient is a 37-year-old woman, ex-smoker, with a previous history of pneumothorax in April 2004 and December 2006, who was diagnosed with LAM by lung biopsy in January 2007. Lung function at that time was: FEV1 2010ml (70.3%), FVC 3470ml (99%) and DLCO 4.63mmol/min/kPa (55.9%). The patient had no renal angiomyolipomas and had never presented chylothorax. After the diagnosis, treatment was initiated with oral progesterone at a dose of 5mg/day, which was maintained for 10 months. The disease progressed, leading to worsened symptoms and declining lung function: FEV1 1540 (54.5%), and the progesterone was suspended after having observed no improvement in January 2008. In June of that same year, it was decided to initiate treatment with the compassionate use of oral sirolimus at a dosage of 2mg/day. At that time, FEV1 was 1230ml (43.4%), FVC 3080ml (88%) and DLCO 3.39mmol/min/kPa (41.1%), and the functional decline that had been observed in 13 months was 780ml in FEV1, 390ml in FVC and 1.24mmol/min/kPa. Twenty-eight months after the start of the therapy, the functional decline has slowed down, and current lung function levels are: FEV1 1050ml (38.01%), FVC 3260 (95%) and DLCO 4.16mmol/min/kPa (50.7%) (Table 1). The only side effects presented were oral ulcers and brittle nails.
DiscussionThis present study analyzes the effects of treatment with sirolimus (rapamycin) in three patients with LAM, two of whom affected with sporadic LAM and one with LAM associated with TS.
For years, the treatment of LAM has been centered on the use of anti-estrogenic therapies. The fact that it affects women almost exclusively and can become exacerbated during pregnancy or when taking contraceptives and that estrogen receptors have been identified in the lung tissue have advocated the use of different therapeutic options, such as oophorectomy and treatment with tamoxifen, progesterone and gonadotrophin-releasing hormone analogues, with variable and partial results. There are published series of cases that report improvement in lung function after the administration of progesterone.2,3 More recent studies, such as that of Taveira-Dasilva et al., have respectively analyzed 275 patients with LAM, comparing the FEV1 and DLCO of the patients who had been treated with intravenous progesterone or with oral progesterone with the levels of those patients who had received no treatment. Taking into account the limitations of a retrospective study, the authors conclude that progesterone did not slow the decline in lung function in LAM.4 Despite the fact that there has been no placebo-controlled clinical assay on treatment with progesterone carried out in LAM, this is still the most widely used therapeutic option. Nevertheless, the guidelines of the European Respiratory Society for the diagnosis and management of LAM do not recommend the routine use of progesterone, except in those patients who manifest a rapid decline in symptoms and lung function.5
Lung transplantation constitutes the only therapeutic option when the disease progresses. A recent paper observed a mean survival after lung transplantation in LAM of 86% after one year, 76% after two years and 65% after five years.6
There is a close relationship between LAM and tuberous sclerosis complex (TSC). TS is a dominant autosomal disease, characterized by a mutation in one of the genes: TSC1 or TSC2.7 The genes codify two proteins: hamartin (TSC1) and tuberin (TSC2), which regulate cell size and growth. Sporadic LAM is associated with somatic mutations in the TSC genes. The TSC1 and TSC2 proteins regulate their signal through mTOR (mammalian target of rapamycin), controlling cell growth, the cell cycle apoptosis and autofagia.8
“LAM cells”, atypical smooth muscle cells that are characteristic of LAM, show mutations in these genes (TSC1 and TSC2), which lead to a deficiency of the hamartin–tuberin proteins.9 The loss of these proteins produces an anomalous activation of mTOR, giving rise to uncontrolled cell growth.
Sirolimus, an immunosuppressant approved by the FDA, could mimic the function of tuberin in patients with LAM, impeding the anomalous activation of mTOR, as has been demonstrated in cell and animal models. It is known to inhibit the proliferation of smooth muscle cells in the coronary arteries after stent placement. Preclinical studies have demonstrated that treatment with sirolimus analogues (CCI-779) reduces the size of the tumors in animal models (mice) with TS.10 Given the efficacy and safety of sirolimus in preclinical studies and its potential beneficial effects, studies have been designed in humans.
Renal angiomyolipomas are benign tumors that are rich in fat, muscle tissue and blood vessels and are present in 40% of the patients, both in sporadic LAM as well as in LAM associated with TS.11 They are associated with mutations in the TSC1 and TSC2 genes. Clinical cases have been reported with reduction in the size of renal angiomyolipomas after treatment with sirolimus.12 In 2008, results were published of the first clinical assay in phase II with sirolimus for the treatment of renal angiomyolipomas in patients with TS and in patients with LAM.13 The study included 25 patients: 6 with sporadic LAM, 12 with TS and LAM and 7 with TS without LAM. After 12 months of treatment with sirolimus, there was a reduction of 53.2±26.6% in the mean volume of the angiomyolipomas compared with the baseline volume. When the lung function variables were analyzed in the 11 patients with LAM, mean increases were observed in FEV1 of 118±330ml and in FVC of 390±570ml after 12 months of treatment. Eight of the 11 patients had an increase of at least 250ml in FVC during the year of treatment with sirolimus. These beneficial effects tended to revert after the withdrawal of the drug. Among the most frequent adverse effects were oral ulcers, diarrhea and infections of the upper respiratory tract, as in the cases presented, two of which presented oral ulcers. The authors conclude that the treatment with sirolimus for one year reduces the size of renal angiomyolipomas and can improve the lung function in patients with TS and LAM.
In this paper, it was observed in the second and third cases that treatment with sirolimus slowed down the fall in lung function, and in the first case a significant improvement was seen. Thus, after two years of treatment with sirolimus, the first patient's FEV1 improved by 870ml and the second one's by 30ml, while the third lost only 80ml in FEV1. Nevertheless, it is necessary to wait for the results of the randomized, double-blind, placebo-controlled MILES trial (The Sirolimus Multicenter International Lymphangioleiomyomatosis Efficacy and Safety trial), done in the United States in phase III, in order to discern whether sirolimus actually delays the loss in lung capacity of LAM patients.
Please cite this article as: Casanova Á, et al. Tratamiento de la linfangioleiomiomatosis con sirolimus. Arch Bronconeumol. 2011;47:470–2.