In recent years, the number of lung transplantations performed as the last option for many respiratory diseases has grown considerably, both in adults and children. However, the causes for the relatively short survival of lungs compared to other organ transplants still need to be studied.
Techniques have improved since the 1950s when experimental lung transplantation began, and the different animal species used now include rodents. The advantage of using these small species is that the surgical model has been expanded and standardized, and different respiratory problems can be studied.
In this review we examine the different technical strategies used in experimental transplantation in rats and mice, focusing on surgical techniques and anesthesia and monitoring methods, and highlighting the major contributions of mouse lung transplantation to the field.
A lo largo de las últimas décadas, el número de trasplantes pulmonares realizados como terapia final de muchas enfermedades respiratorias ha ido creciendo considerablemente, tanto en la población adulta como a nivel pediátrico. Sin embargo, se hace muy necesario estudiar las causas por las que su supervivencia es relativamente baja en comparación con otros trasplantes de órganos.
Por ello, desde mediados del siglo pasado comenzaron a realizarse trasplantes pulmonares experimentales, cuya técnica ha ido mejorando, y se ha ampliado a distintas especies animales hasta llegar a los roedores. La ventaja que presentan estas especies pequeñas ha facilitado que el modelo quirúrgico se haya extendido y estandarizado, permitiendo estudiar diferentes aspectos relacionados con las enfermedades respiratorias.
En esta revisión se analizan las distintas modalidades técnicas disponibles de trasplante experimental en rata y ratón, destacando tanto la técnica quirúrgica como la anestésica o la monitorización, así como las principales aportaciones generadas por el trasplante pulmonar murino.
Lung transplantation is occasionally the only therapeutic option available in the final stages of respiratory diseases such as chronic obstructive pulmonary disease, diffuse interstitial pulmonary disease, bronchiectasis associated with cystic fibrosis, bronchiolitis obliterans, sarcoidosis or arterial pulmonary hypertension.1–3
Since the first transplantations were performed in animals in 1940,4,5 these experimental models have contributed to the development of surgical procedures and the prevention of associated complications, such as rejection or ischemia–reperfusion injury. Although the first mouse transplantation took place in 1971,6 in the 20th century the procedure was generally performed in dogs,4,5 pigs, sheep7 and monkeys,8–10 since larger animals offer advantages in the development of surgical techniques and transfer of results to humans in the clinical setting. These species continue to be used today, particularly for surgical training, but smaller species, such as rabbits,11 rats and mice, which have considerable advantages in terms of expense, management and logistics, and are less constrained by ethical considerations, have acquired greater prominence.
Orthotopic transplantation of the left lung was initially developed in rat models,6 while heart–lung transplant is less common due to high mortality rates.12 Improvements in surgical and anesthetic techniques, mechanical ventilation and preservation and reperfusion of organs have improved the success rate and reproducibility of the model. Heterotopic transplantation models have also been developed, although ventilation and good graft perfusion have not been achieved.13,14 Finally, the mouse model is enhanced by the availability of a wide range of strains that allow the study of genetic factors that may affect the transplant.15,16
The aim of this review is to describe the different procedures used in mouse lung transplantation, paying special attention to surgical techniques and perioperative management, including anesthesia–analgesia, mechanical ventilation, post-operative care, and prevention of complications. We also review the main clinical contributions of lung transplantation in rodents.
Anesthesia and Perioperative CareAnesthetic protocols must provide sufficient anesthesia and analgesia to maintain the physiological stability of the animal, and they must be easily reversible after surgery is completed. Although induction and recovery from inhalation anesthesia16 (1.5%–2% isoflurane or 2.5%–3% sevoflurane, both known to protect the lung17) is rapid, this technique can be difficult to apply in the absence of proper facilities. Specifically, considerable economic resources are called for, as inhalation anesthetics are costly, and a team specializing in mechanical ventilation must be available to administer the anesthetic gases. When inhalation anesthesia is not an option, parenteral methods can be used instead (Table 1).
Anesthestic Combinations Used in Rats and Mice During Surgical Procedures, Including Lung Transplantation.
| Protocol | Dose (mg/kg) | Duration (min) | |
|---|---|---|---|
| Rat | Mouse | ||
| Ketamine+medetomidine | 75–100+0.25–0.5 | 75–150+0.5–1 | 40–50 |
| Ketamine+xylazine | 75–100+12 | 75–150+12–20 | 40–50 |
| Ketamine+diazepam | 80+20 | 75–100+5 | 30–40 |
| Medetomidine+fentanyl | 0.3+0.3 | – | 50–60 |
| Atipamezolea (α2-adrenergic antagonist) | 0.5–1 | 0.5–1 | – |
Ketamine is one of the most widely used drugs. It provides an analgesic effect which is enhanced when administered together with an α2-adrenergc receptor antagonist, such as xylazine or medetomidine, which simultaneously reduces muscle rigidity. This combination of anesthetics can be easily reversed with the administration of an α2-adrenergic receptor antagonist, such as atipamezole. Other combinations include ketamine and the benzodiazapine diazapam, or fentanyl combined with medetomidine.
Diuresis caused by α2-adrenergic receptor antagonists must be compensated with subcutaneous fluid replacement. Ophthalmic ointment must also be applied to prevent corneal dryness.
Thoracotomy is a potent nociceptive stimulus, so appropriate analgesia is essential. Analgesia is generally provided in the form of preoperative subcutaneous administration of opioids (e.g., buprenorphine, 10–50μg/kg/8h in rats or 50–100μg/kg/8h in mice) combined with non-steroidal anti-inflammatory drugs (meloxicam 2mg/kg/day). Finally antibiotic prophylaxis with cefazolin (30mg/kg) or ceftriaxone (70mg/kg) is recommended.
VentilationOnce anesthetized, the animals must be intubated with 14 G catheters for rats >300g, 16 G for rats 200–300g or 22 G for mice weighing around 25g, and mechanically ventilated. For the recipient, the pressure-controlled mode is usually recommended, since the left lung will not be functional during a large part of the surgery.
The ventilator should be set to a tidal volume of 7–10ml/kg (if volume-controlled ventilation is used), or a maximum pressure of 10–12cmH2O (in the case of pressure-controlled ventilation), with a respiratory rate of 60–100breaths/minute. Moreover, a positive end-expiratory pressure of 2–5cmH2O is highly recommended for avoiding the formation of atelectasis during expiration.
Intraoperative MonitoringEquipment specifically designed for rodents is available for recording pulse oximetry, capnography, non-invasive arterial pressure or electrocardiogram. Devices designed for humans or large animals are generally unsuitable for rodents.
It is also important to monitor body temperature using a thermometer or rectal probe and to maintain it between 37.5 and 38.5°C. Hypothermia is a very common complication in rodents and adverse events can occur if temperatures fall below 32°C, including delayed recovery from anesthesia. An electric blanket or hot water bottle can be used to maintain body temperature, although the animal must be monitored to avoid possible hyperthermia. Oxygen saturation tends to remain at levels above 99% when the inspired fraction of oxygen is increased to between 30% and 60%, compared to 21% ambient air.
Surgical Aspects of Orthotopic Lung Transplantation in Mice and RatsThe left lung orthotopic transplantation model is the most widely used, thanks to its viability and greater simplicity, since the left lung is formed of a single lobe, while the right lung is divided in 4. However, orthotopic transplantation of the right lung has also been performed in mice.
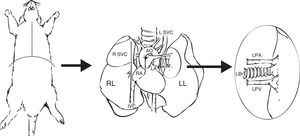
Preparation of the DonorExtraction of the complete heart–lung block is generally recommended. The animal is anesthetized and placed in a supine position, and the operative field is prepared by shaving the skin of the chest and applying povidone iodine or chlorhexidine in an outward movement from the center of the chest. A median sternotomy is then performed with inferior vena cava, subcutaneous or intracoronary heparin delivery (100IU/100g) to prevent clots forming in the lung for transplant. Using an optical microscope, the lung hilum is dissected to locate the aortic arch, which is then clamped. Incisions are simultaneously made in the atria and the inferior vena cava.
In most models, anterograde perfusion is performed via the right ventricle19,20 or the pulmonary trunk15,21–23although it is advisable to combine this procedure with retrograde perfusion from the left atrium22 to simulate physiological conditions. The lungs may be perfused at a constant, uniform pressure (10–15cmH2O) with cold preservation fluid (4°C), consisting of low potassium dextran glucose (LPDG).
The trachea is then ligated and dissected for extraction of the entire heart–lung block, with the lung inflated to prevent the formation of atelectasis and to improve oxygenation and the presence of surfactant in order to reduce ischemia–reperfusion injury.24,25 Recommendations for inflation range between 50%22,25 and 75%–100%26 of total lung capacity, taking care to avoid barotrauma. Inflation before preservation also reduces the expression of proinflammatory markers after reperfusion.26
For preservation, the donor lung is generally maintained at 4°C to minimize ischemic injury to the graft.27 The most commonly used preservation solution is low potassium dextran (Perfadex),16,28–30 although others are available, including Celsior,31 Euro-Collins or Wisconsin.24
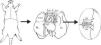
Preparation of the GraftWhen the lung block has been extracted, the left lung must be separated, clamping the bronchus to keep it inflated. The bronchus, the artery and the pulmonary veins are dissected as distally as possible to the lung to assist anastomosis (Fig. 1).
Extraction and preparation of the donor lung. Median sternotomy and dissection and extraction of the heart–lung block. Dissection of the left pulmonary artery (LPA), left pulmonary veins (LPV), and the left bronchus (LBr). AO: thoracic aorta; IVC: inferior vena cava; LA: left atrium; L SVC: left superior vena cava; LL: left left; RA: right atrium; R SVC: right superior vena cava.
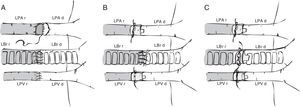
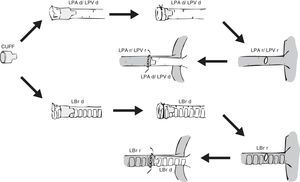
Preparation of the left lung will vary depending on the anastomosis method used: vascular and bronchial anastomosis with sutures,6,19,21,32 bronchial anastomosis with sutures and vascular anastomosis with cuffs,30,33–36 and vascular and bronchial anastomosis with cuffs20,22,23,35–39 (Fig. 2). In the first case, the lung hilum is dissected, separating each structure for anastomosis, while maintaining the lung in the low temperature preservation medium. In the latter 2 cases, rather than using sutures, the vascular and bronchial anastomoses are simplified by using cuffs designed for transplantation of lungs33 and other organs.40–42 These are ring-shaped devices made of polyvinyl chloride or Teflon®, prepared from cannulae or catheters. Sizes for rats range from 18G to 14G, the larger ones being used in the bronchus and the smaller ones in the pulmonary artery.22 Conventional cuffs consist of a main body, on which the vessels or the bronchus are joined and an extension tab to facilitate manipulation (Fig. 3). The cuff is 2–3mm in length (body 1, 1.5 or 2mm and the tab 1 or 1.5mm). A modified, tabless cuff model has been developed,32,36,42 but this makes it more complicated to manipulate. For this reason, some groups use the standard cuff, and remove the tab after anastomosis has been performed.33
Surgical techniques in mouse model lung transplantation. (A) Vascular and bronchial anastomosis with sutures. (B) Bronchial anastomosis with sutures and use of vascular cuffs in the pulmonary vein and artery. (C) Vascular and bronchial anastomosis using cuffs. LBr d: left bronchus of donor animal; LBr r: left bronchus of recipient animal; LPA d: left pulmonary artery of donor animal; LPA r: left pulmonary artery of recipient animal; LPV d: left pulmonary vein of donor animal; LPV r: left pulmonary vein of recipient animal.
Placement of a cuff in the donor lung and anastomosis with the recipient lung. LBr d: left bronchus of donor animal; LBr r: left bronchus of recipient animal; LPA d: left pulmonary artery of donor animal; LPA r: left pulmonary artery of recipient animal; LPV d: left pulmonary vein of donor animal; LPV r: left pulmonary vein of recipient animal.
Irrespectively of the type of cuff used, the procedure is conducted by introducing the vein, artery or bronchus of the donor lung through the hole of the cuff. It is then everted over the cuff and fixed to the cuff with a polypropylene ligature, 7/0 or 8/0 in rats22,23 and 10/0 in mice.18 The bronchus is occasionally prepared at the moment of implantation to prevent preservation solution from infiltrating the airway.18,20
Recipient PreparationThe animal is anesthetized, given appropriate analgesia and placed in right lateral decubitus position. The surgical field is prepared in the same way as for the donor animal and a lateral thoractomy is made through the fourth intercostal space. The left lung is extracted and the principal structures are isolated, clamping the left pulmonary artery, vein and bronchus, as distally as possible to the lung. These structures are dissected as close as possible to the pulmonary parenchyma. To avoid the formation of thrombi, heparinized saline (0.5–1IU/ml) is administered to the artery and vein. All structures must be handled carefully to avoid vascular tears.
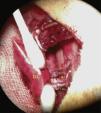
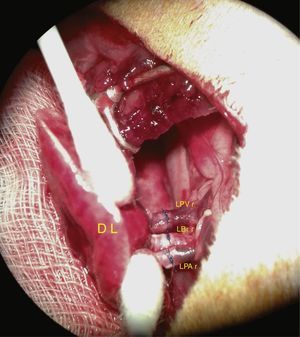
The vascular structures and the bronchus are then anastomosed. Cuff anastomosis is shown in the photograph in Fig. 4.
When the bronchus has been anastomosed, the donor lung must be ventilated to avoid the formation of atelectasis and to enhance alveolar recruitment. Rapid lung reperfusion indicates that the anastomosis is patent. Finally, the native lung is extracted from the recipient animal, leaving only the donor lung.
A drainage tube must be inserted so that remains of blood, fluid or air around the lungs can be eliminated and the lungs can expand normally. The chest cavity is then closed by layers, and the drainage tube is withdrawn when the animal recovers from anesthesia and begins to breathe spontaneously.
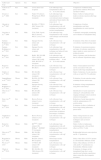
The time to complete surgery varies, depending on the group of professionals and the technique used. In general, the procedure is faster with the cuff technique than with conventional sutures, particularly when sutures are applied to vessels and the bronchus. Similarly, the survival rate is higher when anastomosis is performed with cuffs, compared to sutures (Table 2).
Comparison of Different Mouse Model Lung Transplantation Protocols.
| Technique | Surgery Time (min) | Type of Anastomosis | Order of Anastomosis | Cuff Length (mm) | Cuff Diameter (mm) | Survival (%) |
|---|---|---|---|---|---|---|
| Suture | ||||||
| Asimacopoulos,6 1971 | Not described | Suture | B-A-V | – | – | 50 |
| Marck,32 1982 | 240 | Suture | V-A-B | – | – | 80a 50b |
| Prop,19 1984 | 210±31 and 238±19 | Suture | V-A-B | – | – | 89 and 75 |
| 7521+2008 | 78.4±5.2 | Suture | B-A-V | – | – | 89a 50b |
| Vascular cuffs and bronchial suture | ||||||
| Mizuta,33 1989 | 101±4.8 | Conventional cuff | V-A-B | 2 (body: 1; tab: 1) | 2 and 1.65 | 88.8 |
| Kubisa,34 2003 | 120 | Conventional cuff | V-A-B | Not described | 0.8 | 96.57 |
| Mizobuchi,35 2004 | 116±1.6 | Conventional cuff | V-A-B | 4 (body: 2.5; tab: 1.5) | 1.65 | 95.6 |
| Zhai,36 2008 | Not described | Modified cuff | A-B-V | 2.5 (body: 1; tab: 1.5) | 1.65 | Not described |
| Habertheuer,30 2013 | 130±9 | Conventional cuff | B-A-V | Not described | Not described | 100 (70–90–100) |
| Vascular and bronchial cuffs | ||||||
| Mizuta,37 1991 | 64.1±3.6 | Not described | Not described | Not described | Not described | 90b |
| Reis,38 1995 | 108.6±4.2 | Conventional cuff | A-V-B | 3 (body: 2; tab: 1) | 1.65 | Not described |
| Santana Rodríguez,23 2004 | 110±12.7 | Conventional cuff | V-B-A | 3 (body: 1.5; tab: 1.5) | 2.2 and 1.65 | Not described |
| Mizobuchi,35 2004 | 84.8±0.6 | Conventional cuff | V-B-A | 4 (body: 2.5; tab: 1.5) | 2.1 and 1.65 | 91.8 |
| Zhai36, 2008 | Not described | Modified cuff | A-B-V | 2.5 (body: 1; tab: 1.5) | 1.65 | Not described |
| Sugimoto,39 2009 | 110 approx. | Not described | A-B-V | Not described | Not described | 93.6 |
| Jungraithmayr,16 2009 | 95.6±8.5 | Conventional cuff | A-B-V | Different sizes | 0.64–0.51–0.4 | 80 |
| Santana Rodríguez,22 2011 | 107.7±6.2 | Conventional cuff | A-B-V | 3 (body: 1.5; tab: 1.5) | 1.65 | 87.6 |
| Zheng,43 2013 | 96.8±6.2 | Conventional cuff | A-V-B | Different sizes | 0.81–0.64–0.51 | 96.7 |
| Zheng,43 2013 | 85.3±6.9 | Modified cuff | A-V-B | Not described | 0.81–0.64–0.51 | 80 |
Order of anastomosis: A: artery; B: bronchus; V: vein.
After surgery, analgesic and antibiotic treatment must be administered for at least 5–6 days, and analgesia can be increased in case of pain. The surgical wound must be cleaned and disinfected to aid healing. Protocols for monitoring and assessment of endpoint criteria must be established, including aspects such as loss of weight, physical appearance, ventilatory pattern, presence of cyanosis, or the behavior of the animal. If any of these variables changes, corrective actions must be immediately implemented.
Perioperative Complications and ManagementThe most significant complications occur as a result of the surgical procedure, in the preparation of both the donor and the recipient.44 A relatively long learning curve is required before skills are acquired in the use of microsurgical equipment and success in the technique is achieved, and mortality can be high during the training period.15 Some authors set 50 transplantations as the threshold for perfecting the procedure.20 The duration of the surgery and time spent performing anastomoses impact on the appearance of complications and on the animal's survival.6,16,30,32,44
In general, the use of cuffs requires less skill and experience, and the procedure is faster than with sutures.38,45 Vascular cuffs reduce the risk of bleeding and with it the formation of granulation tissue, reducing the long-term risk of thrombosis or stenosis in the anastomosis sites.38 Bronchial leaks, a factor causing pneumothorax which compromises the functionality of the graft, are rarer with cuff anastomosis.35 However, the diameter of the cuff must be adequate to avoid reducing the size of the lumen, and moreover, a foreign-body reaction may occur.35,38,45 Modified cuffs, without extension handles or tabs, reduce the risk of twisting of the vessels or bronchi after anastomosis and injury caused by these extensions themselves. They are also smaller, so less foreign-body reaction is produced.36 Other perioperative complications include vena cava rupture, pleural empyema,34 respiratory arrest,38 and venous thrombosis.43
The most significant postoperative complications include respiratory failure, hemoptysis, thrombosis of a bronchial vein or artery, pneumothorax, respiratory infections, pleural effusion, pulmonary edema, and graft rejection.19,46
Main Scientific ContributionsDespite the complexity of the surgery, rodent lung transplantation models are sufficiently standardized and reproducible to be useful in the evaluation of different surgical techniques and the mechanisms of the main transplantation complications in humans. To date, mouse lung transplantation has helped generate knowledge on donor characteristics,24,47 lung conservation and preservation models,26,48,49 ischemia–reperfusion injury,48,50–52 and particularly, acute and chronic rejection (Table 3).48,53–66
Summary of Principal Aspects Evaluated in Mouse Model Lung Transplantation.
| Author and Year | Species | Sex | Strains | Model | Objective |
|---|---|---|---|---|---|
| Aeba et al.,49 1992 | Rat | Male | Lewis (donor and recipient) | Left orthotopic lung transplantation after 6 or 12h of cold ischemia | Comparison of different lung preservation solutions (Wisconsin and Euro-Collins) |
| Zweers et al.,47 2004 | Rat | Male | Fisher 344 (donor); Wistar Kyoto WKY (recipient) | Left orthotopic lung transplantation with conventional suture technique and induced brain death in the donor for 6h | Study of effect of induced brain death on chronic rejection after lung transplantation |
| Okazaki et al.,58 2007 | Mouse | Male | BALB/c, C57BL/6 (B6)a (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique and heterotopic trachea transplantation | Comparison of trachea transplantation and lung transplantation in acute rejection study |
| Greschus et al.,53 2009 | Rat | Male | F344, Dark Agouti, WKY (donors); WKY, Lewis, F344 | Left orthotopic lung transplantation with vascular cuff technique and bronchial suture | Volumetric tomography monitoring and evaluation of transplanted lung |
| Santana-Rodríguez et al.,52 2011 | Rat | Male | Sprague-Dawley (donor and recipient) | Left orthotopic lung transplantation with cuff technique | Evaluation of the effect of estradiol on ischemia–reperfusion lung injury |
| Santana Rodríguez et al.,22 2011 | Rat | Male | Sprague-Dawley (donor and recipient) | Left orthotopic lung transplantation with cuff technique and induced brain death in the donor | Evaluation of anastomosis patency and signs of ischemia–reperfusion injury similar to acute rejection in 2 technical models |
| Kreisel et al.,50 2011 | Mouse | Male | Balb/c, B6, B10.BR CD11c-EYFP (donor); B6 LysM-GFP, B6 TNF-α−/− (recipient) | Left orthotopic lung transplantation with cuff technique after 1h and 18h of cold ischemia | Study of primary graft dysfunction due to ischemia–reperfusion injury |
| Kreisel et al.,51 2011 | Mouse | Male | B6 (donor)-B6 (B6), B6 (Bcl3) | Left orthotopic lung transplantation with cuff technique | Study of transcription factor Bcl3 and its association with acute rejection after lung transplantation |
| Dodd-o et al.,59 2011 | Mouse | Male | B6, BALB/c (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Study of reduction of acute rejection with use of anti-CD-154 antibodies |
| Jungraithmayr et al.,55 2012 | Mouse | Male | BALB/c (H-2Kd) (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Evaluation of acute rejection using scanning electron microscopy |
| Santana-Rodríguez et al.,66 2012 | Rat | Male | Sprague-Dawley (donor and recipient) | Left orthotopic lung transplantation with cuff technique | Evaluation of lung transplantation using DNA microarrays |
| Chen et al.,57 2013 | Mouse | Male | B6, BALB/c (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Identification of acute rejection by studying T cell metabolism with imaging techniques (micro-positron emission tomography). |
| Erne et al.,62 2013 | Rat | Male | Brown-Norway (donor); Lewis (recipient) | Left orthotopic lung transplantation with cuff technique | Reduction of acute lung rejection with the administration of N-acetylcysteine |
| Tanaka et al.,26 2014 | Rat | Male | Lewis (donor and recipient) | Left orthotopic lung transplantation with cuff technique after 6h of preservation at different percentages of inflation | Evaluation of effect of inflation of donor lungs during pulmonary preservation. |
| Takahashi et al.,56 2014 | Rat | Male | Brown-Norway (donor); Lewis (recipient) | Left orthotopic lung transplantation with cuff technique | Study of lung function in acute rejection using noninvasive techniques such as forced oscillation |
| Kreisel et al.,61 2010 | Mouse | Male | B6CD45.,1 B6II− (donor); B6, B6Rag−/−, B6/B6II− F1 (recipient) | Left orthotopic lung transplantation with cuff technique and bone marrow transplantation | Study of effect of pulmonary nonhematopoietic cells and their effect on CD4+ T cells |
| Zhou et al.,60 2015 | Mouse | Male | B6, BALB/c (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Relationship between acute rejection and IL-17 production |
| Raissadati et al.,63 2015 | Mouse | Male | BALB/c (donor); B6 (recipient) | Heterotopic trachea transplantation | Study of the effect of CCN1 protein in a bronchiolitis obliterans model |
| Wu et al.,64 2015 | Mouse | Male | C57Bl/10, B6 (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Study of the influence of CD4+ T cells and IL-17 production in a bronchiolitis obliterans model |
| Chuck et al.,54 2016 | Mouse | Male | B6, BALB/c (H-2Kd) (donor); B6 (recipient) | Left orthotopic lung transplantation with cuff technique | Evaluation of changes in the transplanted lung due to acute rejection and ischemia–reperfusion injury using ultra-short magnetic resonance. |
Primary graft dysfunction occurs in the first 72h and severity depends on the PaO2/FiO2 ratio and the presence of radiological infiltrates. It is a significant cause of morbidity and mortality in the immediate postoperative period.
Analysis of this phenomenon in mouse models has led to a better characterization of the causative mechanisms, in which the primary lesion of the neutrophils caused by ischemia–reperfusion appears to play a fundamental role. Thus, neutrophils isolated from the airways of transplant recipient have been seen to stimulate the donor's dendritic cells by a contact-dependent mechanism to induce IL-12 production and to expand alloantigen-specific T cells.50 This phenomenon, in turn, has been shown to be regulated by the degranulation induced by TNF-α associated with the neutrophil plasma membrane.50 Furthermore, some factors which modulate this cell-mediated response have been identified, which may be of potential interest in the design of future therapeutic approaches. The transcriptional factor Bc13 has been seen to limit granulopoiesis via a dependent NF-kB pathway.51
Acute Cell-mediated RejectionMouse models provide an ideal medium for the study of acute cell-mediated rejection, as the grade of concordance or discordance of the major histocompatibility antigen can be evaluated in pure strains. This is of particular interest in the development of specific monitoring systems for this transplantation model. In addition to using bronchoalveolar lavages for the study of airway cellularity and measuring oxygenation capacity with arterial blood gases, specific imaging techniques have been developed, such as volumetric tomography53 or ultra-short magnetic resonance.54 These, together with the histological evaluation of the ultrastructural changes in acute rejection using scanning electron microscopy,55 improve the monitoring of the transplanted lung.
Complementary procedures have also been developed for the evaluation of lung function. The forced oscillation technique is very useful in mouse models, since dynamic elastance and its frequency dependence appear to have the ability to predict the grade of acute rejection,56 and can be used as non-invasive indices for detecting and monitoring disease progression. In an image-function setting, micro-positron emission tomography can be used to identify increased 18fluorodeoxyglucose uptake by neutrophils and CD8 lymphocytes,57 providing a more precise picture of acute rejection from the evaluation of T cell metabolism.
The evaluation of the pathogenic mechanisms of acute rejection in mouse models has fostered the development of interventions for prolonging graft survival, for example, by blocking anti-CD28-B7 costimulators and the CD40–CD40 ligand.58 Other authors have shown in an MHC-mismatched orthotopic mouse lung transplantation model that monotherapy with anti-CD154 antibodies may be sufficient to attenuate acute rejection59 by blocking CD154/CD40 costimulation, which fosters a shift in the graft environment toward a predominance of CD4(+) CD25(+) Foxp3(+) regulatory T cells.60 Some pulmonary nonhematopoietic cells have also been reported to play an important role in the in vivo suppression of CD4(+) T cell immune-mediated responses.61
Although it still early, these findings may be transferred to clinical practice in the form of N-acetylcysteine therapy. The administration of this compound in mouse models has been shown to diminish acute lung rejection by reducing the macrophage and T cell infiltration that is intimately related with reduction of the NF-kB-mediated inflammatory signaling pathway.
Chronic RejectionChronic rejection usually manifests as the development of bronchiolitis obliterans, caused by lymphocyte and neutrophils cell infiltration attributable to a series of factors, such as excessive activation of the innate immune system, abnormal angiogenesis, or inadequate epithelial regeneration and remodeling of the lymphoproliferative tissue.48
Although each animal model has its advantages and limitations in the study of bronchiolitis obliterans, the mouse model of bronchiolitis obliterans associated with lung transplantation mimics more closely chronic rejection in humans than other models of bronchiolitis obliterans induced by chemical agents. Moreover, it can be used to make serial evaluations of both lung function and multiple morphological changes or altered cell function, and for testing the efficacy of different drugs. Another important advantage is the availability of models with knock-out and transgenic strains of multiple human diseases, that can be used to reproduce situations of chronic rejection relatively similar to those that occur in different end-stage respiratory diseases, and for evaluating the contribution of potential signaling pathways.
However, some anatomical and physiological factors of the mouse lung cause translational difficulties. Compared to human lungs, mouse bronchioles have fewer submucosal glands and a greater number of Clara cells. This environment protects against bronchiolar epithelial injury, primarily due the production of Clara cell secretory protein and a solution similar to lung surfactant, so more vascular injury than epithelial injury is generated in these rejection models.58 Nevertheless, significant advances have been made in recent years in the development of different bronchiolitis obliterans mouse models63–65 and DNA microarrays for evaluating chronic rejection after transplantation.66
In conclusion, techniques in mouse lung transplantation have developed significantly, contributing to a range of different surgical alternatives with excellent viability of transplanted animals. These experimental surgical models that have demonstrated their utility in generating knowledge on the pathogenic mechanisms of ischemia–reperfusion injury and rejection are now more widely accessible, and may be translated to humans in the medium term.
FundingThis project received assistance with grant FPU2013-01638, awarded to Daniel Ruiz-Pérez by the Spanish Ministry of Education, Culture and Sport. The grant provider played no part in study design, data collection and analysis, or the preparation of the manuscript.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Ruiz-Pérez D. Aspectos técnicos y utilidades del trasplante pulmonar experimental murino. Arch Bronconeumol. 2016;52:596–604.