Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) readmission contributes considerably to the worse outcomes for COPD patients. Predictors for readmission include some socio-demographic variables and the severity of the underlying disease, however, few evidence suggested whether persistently heightened airway or systemic inflammation was related to recurrence of AECOPD. The aim of this study was to evaluate the role of airway and systemic inflammatory biomarkers during AECOPD on predicting readmission for AECOPD.
MethodsConsecutive hospitalized patients with AECOPD were recruited. Inflammatory and clinical indices were evaluated at the day of admission before starting therapy and the day of planned discharge (day 10–14). Predictors for readmission were assessed by binary logistic regression model.
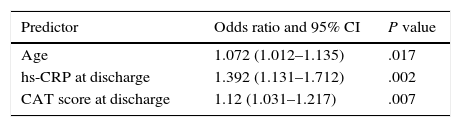
Results93 patients were included with 51 patients (54.8%) were readmitted due to AECOPD at least once during 1 year following the index admission. The logistic regression model indicated that age (OR=1.072, 95% CI: 1.012–1.135, P=.017), hs-CRP (high sensitive-C reactive protein) at day 14 (OR=1.392, 95% CI: 1.131–1.712, P=.002), CAT value at day 14 (OR=1.12, 95% CI: 1.031–1.217, P=.007) were the independent variables statistically significant in predicting rehospitalization.
ConclusionSystemic inflammatory marker CRP was a better predictor of readmission than sputum inflammatory markers. CAT score and age were also useful to predict readmission.
Las rehospitalizaciones por exacerbación aguda de la enfermedad pulmonar obstructiva crónica (EA-EPOC) contribuyen considerablemente a la mala evolución de los pacientes que padecen EPOC. Los factores pronósticos de rehospitalización comprenden variables sociodemográficas y el grado de gravedad de la enfermedad de base. Sin embargo, hay pocos indicios de que exista relación entre la persistencia de la inflamación de las vías aéreas o la inflamación sistémica y la recurrencia de la EA-EPOC. El propósito de este estudio fue evaluar el de papel los marcadores de inflamación sistémicos y de las vías aéreas durante la EA-EPOC como factores pronósticos de rehospitalización por EA-EPOC.
MétodosSe seleccionaron pacientes consecutivos hospitalizados con EA-EPOC. El día del ingreso hospitalario, antes de iniciar el tratamiento, y el día del alta programada (día 10-14), se evaluaron los índices de inflamación y los signos clínicos. Los factores pronósticos de rehospitalización se analizaron mediante un modelo de regresión logística binaria.
ResultadosEl estudio incluyó 93 pacientes, 51 de los cuales (54,8%) fueron rehospitalizados por presentar al menos una EA-EPOC durante el año siguiente a la hospitalización índice. El modelo de regresión logística indicó que la edad (OR=1,072, IC 95%: 1,012-1,135, p=0,017), la concentración de proteína C reactiva de alta sensibilidad (PCR-as) del día 14 (OR=1,392, IC 95%: 1,131-1,712, p=0,002) y la puntuación de la prueba de evaluación de la EPOC (CAT) del día 14 (OR=1,12, IC 95%: 1,031-1,217, p=0,007) eran las variables independientes que pronosticaban la rehospitalización con significación estadística.
ConclusiónEl marcador sistémico de inflamación PCR fue mejor factor pronóstico de la rehospitalización que los marcadores de la inflamación del esputo. La puntuación CAT y la edad también fueron factores pronósticos útiles de rehospitalización.
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide. Acute exacerbation of COPD (AECOPD) is characterized by deteriorating respiratory symptoms beyond day-to-day variations, and leads to a change in medication.1 These episodes contribute considerably to the increased morbidity, mortality and healthcare costs associated with this condition. Some patients are prone to frequent exacerbations and have worse health status,2 greater limitation in daily activities3 and faster disease progression.4 COPD is an important cause of hospitalization, and 63% of patients are readmitted at least once5,6 within the 12 months following admission for exacerbation. Of these, up to 79% present with acute hypercapnic respiratory failure.7 These repeat admissions were mainly due to recurrent exacerbations. Known risk factors for readmission include frequent exacerbation and more severe COPD.6 Early identification of patients likely to have a recurrent exacerbation may permit early implementation of appropriate preventive strategies. Inflammation is a prominent feature of COPD.8,9 Recent studies have shown that COPD is associated not only with an abnormal inflammatory response of the lung, but also with systemic inflammation, including systemic oxidative stress, activation of circulating inflammatory cells, and increased circulating levels of inflammatory cytokines.10 Exacerbation of COPD is generally considered to reflect a flare-up of these underlying inflammatory processes.11
A recent study showed that higher levels of plasma CRP at day 14 post-exacerbation were related to recurrent exacerbations within 50 days.12 It is unknown whether these recurrent exacerbations are also associated with persistence of heightened airway inflammatory status. Frequent exacerbations and severe AECOPD that result in hospitalization lead to worse health status and decreased quality of life.2,13 Few studies on the effect of recovery of quality of life following an AECOPD on readmission for AECOPD have been published. The present authors hypothesized that health status, non-recovery, and persistent heightened inflammatory status may be related to readmission with a recurrent exacerbation. Therefore, a prospective study in a well-characterized cohort was performed, in which airway and systemic inflammation together with quality of life were assessed at admission, prior to treatment, and also at discharge (days 10–14). The relationship between these indices both at admission and at discharge, with re-admission for AECOPD occurring within 1 year of discharge, were also assessed.
MethodsPatient EnrollmentBetween April 1, 2009 and September 30, 2011, a prospective study was conducted in consecutive patients presenting AECOPD admitted to the general ward of the respiratory department of a tertiary hospital in Peking, China. For patients admitted more than once during the study period, only the first admission was included in the analysis. Only admissions of more than 24h duration were included. Inclusion criteria were: COPD diagnosis and presence of exacerbation according to the Global Initiative of Chronic Obstructive Lung Disease definition.14 The stable-stage post-bronchodilator spirometric values recorded in the patients’ medical history in the 6 months prior to study inclusion were used to diagnose COPD. Exclusion criteria were: asthma (excluded by bronchodilation test and history); bronchiectasis (excluded by high-resolution CT); pneumonia; cancer; sleep apnea syndrome; or other forms of active lung disease; hospitalization for reasons other than COPD exacerbation including acute coronary syndrome; congestive heart failure; need for intubation; length of stay (LOS) longer than 30 days; long-term oral corticosteroid (CS) therapy (more than 3 months treatment with 7.5mg per day of prednisone or equivalent); patients who had received systemic CS for their exacerbation for more than 48h before presentation; relapse within 14 days of initial presentation15; patients who died without being readmitted for an AECOPD during the follow-up period. Written informed consent was obtained from all patients, and the Ethics Committee of the hospital approved the study protocol.
Clinical VariablesVariables were collected at admission, and included demographic data, stable-state spirometric values, comorbid conditions, ongoing management before admission, smoking habits, exacerbation frequency in the previous year, and admission symptoms. Exacerbation frequency in the previous year was based on the number of exacerbations reported by the patient in the year prior to recruitment. Previous studies have shown a good correlation between the number of exacerbations recorded in the medical history and the number of exacerbations remembered by the patient over the same 1-year period.16 According to the definition of Hurst et al, frequent exacerbator was defined as exacerbation frequency ≥2 in the previous year, non-frequent exacerbator was defined as exacerbation frequency <2 in the previous year.17 On the day of admission, AECOPD Anthonisen type was determined according to the symptoms presented before starting treatment.7 At admission, at discharge and 8 weeks after discharge (stable status) patients completed the COPD Assessment Test (CAT) based on their symptoms experienced on that day. Classification based on the GOLD 2011 combined assessment of COPD was carried out using the spirometric parameters in stable status, exacerbation frequency, and CAT data obtained in stable status.18
Therapeutic StrategyFollowing GOLD guidelines, patients were treated with nebulized salbutamol, ipratropium bromide and budesonide, intravenous prednisolone at a dose of 30–40mg daily. Duration of intravenous therapy was 4 days. On day 4, patients were switched to an oral tapering schedule of prednisolone. Antibiotics were administered if bacterial infection was suspected (patient-reported sputum purulence) and adjusted according to antimicrobial susceptibilities, when available.
Mechanical ventilation was started by the attending physician for indications such as respiratory arrest, deterioration in level of consciousness, and increasing partial pressure of carbon dioxide (PaCO2), despite optimal pharmacological treatment. Non-invasive ventilation was used initially whenever possible and indicated. Decisions regarding admission or transfer to ICU were taken by the treating unit.
Patients Follow-upPatients were followed from the day of discharge until September 29, 2012 or the day of readmission, if earlier. The primary study outcome was readmission for AECOPD.
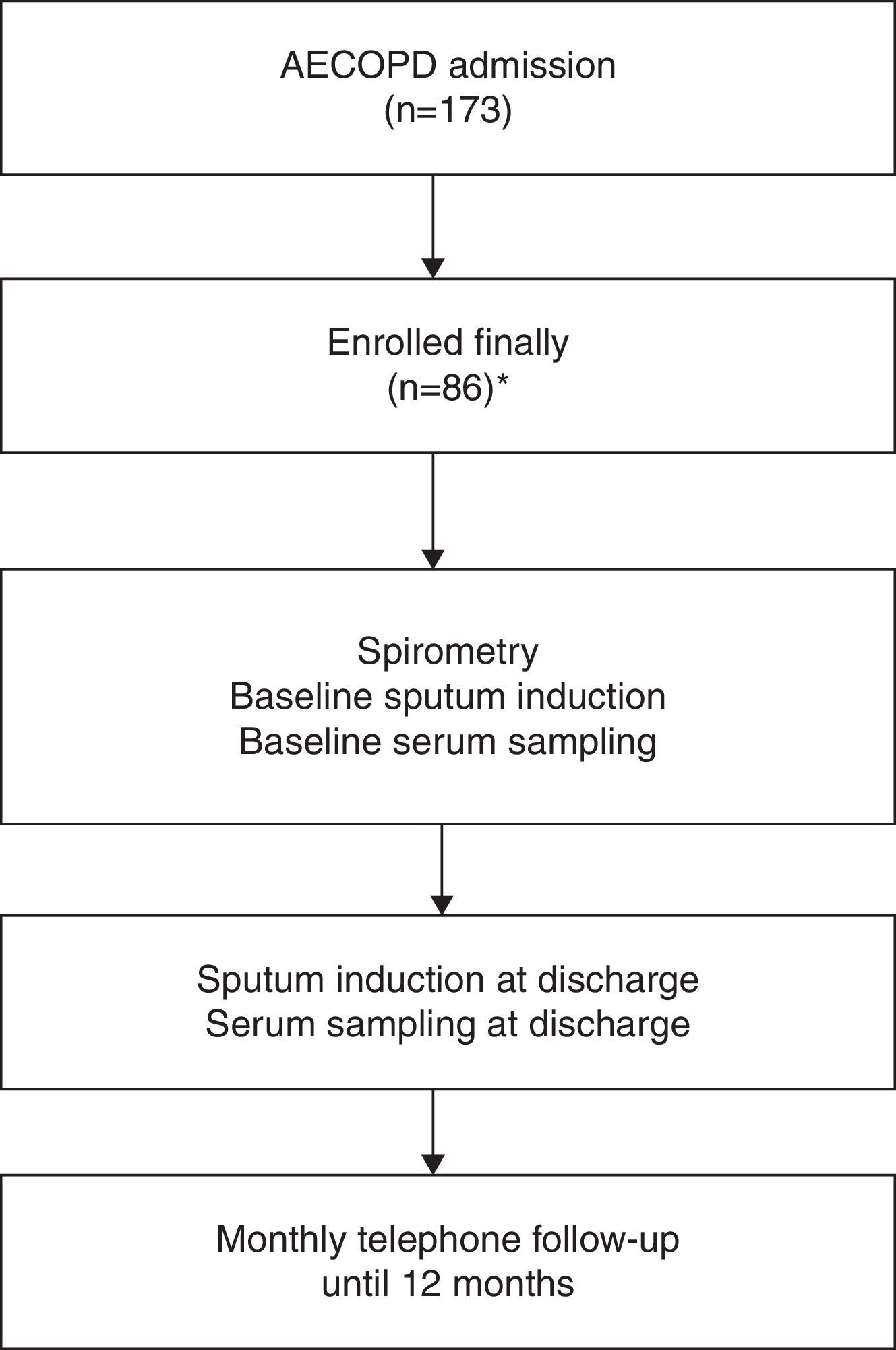
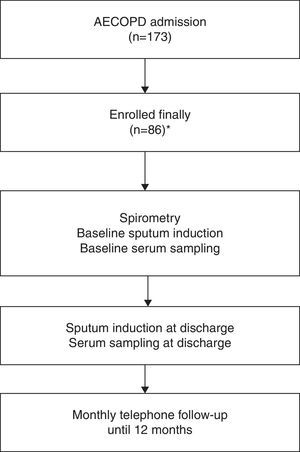
Patients were monitored monthly by telephone to document the occurrence of AECOPD hospitalization. At each telephone contact, a short questionnaire was administered to assess the change in respiratory symptoms and medical interventions in the past month. Patients were also encouraged to report to their attending physicians whenever they experienced symptom worsening. An event-based exacerbation was confirmed if patients experienced at least 1 key symptom worsening plus a change in at least 1 of 3 medications (antibiotics, corticosteroid, and bronchodilator). The end of an exacerbation episode was determined when patients’ symptoms improved and returned to their pre-episode status for at least 3 days, or when the symptoms improved and remained stable for at least 3 days. Readmission within 14 days of the previous discharge was not included in the final analysis, in order to distinguish relapse (symptom fluctuation in the same episode) from recurrence of exacerbations.19,20 The need for hospitalization was determined according to GOLD (Fig. 1).
Patient Sampling and Sample ProcessingPatient sputum and plasma samples were collected on the day of admission, prior to initial treatment and, at discharge (day 10–14). Spontaneous sputum was used; when no spontaneous sputum was available, induced sputum was collected, according to a previous study on the equivalence of induced and spontaneous samples for assessment of airway inflammatory markers.19 Of the sputum samples taken, 97% were spontaneous. Samples was confirmed as valid if there were >25 polymorphonuclear leukocytes and <10 squamous cells present on Gram stain on low power (100×) magnification.21,22 Sputum samples were processed within 2h of collection and divided into two aliquots. One sample was processed with phosphate-buffered saline (PBS),14,23 cytospins were prepared, and cell-free supernatant was collected and stored in aliquots at −80°C pending analysis of soluble mediators. Differential cell counts were performed on May Grünwald Giemsa stained cytospins in a blinded fashion. Quantitative sputum cultures were performed using the second sample, according to previously described methods.12 Bacterial agents were classified as potentially pathogenic microorganisms (PPMs) or non-PPMs. Only PPM growth at thresholds higher than 105 colony-forming units for Streptococcus pneumoniae and 106 colony-forming units for other isolates was considered significant. Peripheral venous blood (7ml) was collected in a Vacutainer tube (BD Diagnostics, NJ, USA) and centrifuged at 6716 G for 10min at 4°C. Plasma was then separated and stored at −80°C until further analysis.
Statistical AnalysisStatistical analysis was performed using SPSS version 11.5.0. Data were not normally distributed and nonparametric statistics were therefore used. Categorical variables are presented as n (%). Numerical variables are presented as median (interquartile ranges) for skewed numerical data. Wilcoxon's signed rank test was used for analysis of paired data, and the Mann–Whitney U test was used for paired group analysis. Categorical outcome variables were analyzed using Fisher probabilities. Statistically significance was set at P<.05.
Exploration of variables independently associated with readmission was conducted using a binary logistic model. Readmission was used as the dependent variable. Factors associated with readmission in the univariate analysis (P<.10) were used in a binary logistic regression predictive model. A P-value of less than .05 in the multivariate model was considered statistically significant.
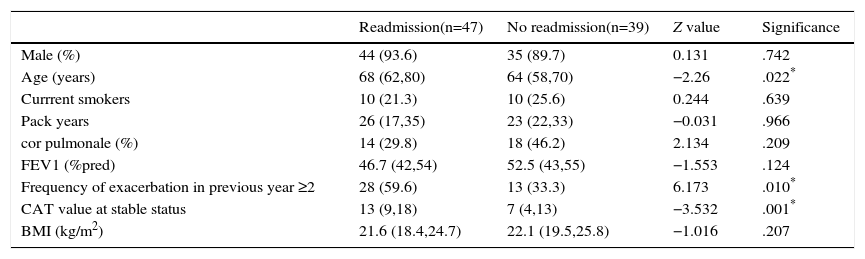
ResultsPatient CharacteristicsA total of 173 eligible patients were included in the study. However, 45 patients were subsequently excluded due to pneumonia (n=6); congestive cardiac failure or left ventricular failure at admission (n=5); pulmonary embolism (n=3); need for intubation at admission (n=14); length of stay (LOS) longer than 30 days (n=5); having received systemic CS for their exacerbation for more than 48h (n=5). Seven patients had “long-term oral corticosteroid use” and were also excluded. Eleven of the 173 patients recruited did not survive the recruitment admission. Twenty-two patients died without having a readmission due to COPD exacerbation during the follow-up period and were excluded from the study of risk factors for a COPD readmission. Nine patients had relapse within 14 days of discharge. Therefore, a total of 86 patients were included in the final analysis; 47 patients (54.7%) were readmitted for AECOPD at least once during the 12 months following the index admission. Table 1 shows the characteristics of the groups. Compared with the no-readmission group, the readmission group comprised older patients; more patients with frequency of exacerbation in previous year ≥2; higher stable-status CAT value; more patients in GOLD group D; and more patients with diabetes.
Characteristics of Patients With Readmission and With no Readmission.
| Readmission(n=47) | No readmission(n=39) | Z value | Significance | |
|---|---|---|---|---|
| Male (%) | 44 (93.6) | 35 (89.7) | 0.131 | .742 |
| Age (years) | 68 (62,80) | 64 (58,70) | −2.26 | .022* |
| Currrent smokers | 10 (21.3) | 10 (25.6) | 0.244 | .639 |
| Pack years | 26 (17,35) | 23 (22,33) | −0.031 | .966 |
| cor pulmonale (%) | 14 (29.8) | 18 (46.2) | 2.134 | .209 |
| FEV1 (%pred) | 46.7 (42,54) | 52.5 (43,55) | −1.553 | .124 |
| Frequency of exacerbation in previous year ≥2 | 28 (59.6) | 13 (33.3) | 6.173 | .010* |
| CAT value at stable status | 13 (9,18) | 7 (4,13) | −3.532 | .001* |
| BMI (kg/m2) | 21.6 (18.4,24.7) | 22.1 (19.5,25.8) | −1.016 | .207 |
| GOLD Group | ||||
|---|---|---|---|---|
| A | 6 (12.8) | 13 (33.3) | 9.668 | .018* |
| B | 2 (4.347) | 3 (7.7) | ||
| C | 10 (21.3) | 9 (23.1) | ||
| D | 29 (61.7) | 14 (35.9) | ||
| Comorbidities | ||||
|---|---|---|---|---|
| Arterial hypertension | 17 (36.2) | 10 (25.6) | 0.604 | .417 |
| Ischemic heart disease | 14 (29.8) | 7 (17.9) | 1.541 | .209 |
| Diabetes | 12 (25.5) | 3 (7.7) | 4.634 | .028* |
| Congestive heart failure | 10 (21.3) | 4 (10.3) | 1.891 | .162 |
| Renal disease | 4 (8.5) | 3 (7.7) | 0.006 | 1.013 |
| Preadmission COPD therapy | ||||
|---|---|---|---|---|
| Chronic oxygen therapy | ||||
| 12 (25.5) | 8 (17.9) | 0.634 | .587 | |
| Steroid treatment | ||||
| Long term ICS treatmenta | 24 (51.1) | 20 (51.3) | 0.004 | 1.004 |
| Anticholinergic drugs | 29 (61.7) | 22 (56)4 | 0.351 | .774 |
| Long-acting beta 2 agonists | 34 (72.3) | 28 (71.8) | 0.103 | .839 |
| Exacerbation characteristic | ||||
|---|---|---|---|---|
| Anthonisen Type I | 32 (68.1) | 28 (71.8) | 0.143 | .736 |
| Cold symptom at presentation | 14 (29.8) | 6 (15.4) | 3.227 | .064 |
| Positive bacterial culture at admission | 18 (38.3) | 14 (35.6) | 0.159 | .672 |
| Positive bacterial culture at discharge | 12 (25.5) | 8 (20.5) | 0.269 | .621 |
| Transfer to the ICU during hospital stay | 0 (0) | 1 (2.6) | 0.031 | .894 |
| Hospital Duration | 6.76 (3.0, 16.0) | 9.10 (3.0, 24.0) | −2.394 | .017 |
Data are presented as median (interquartile ranges) for numerical variables or as number (%) for categorical variables. Abbreviations: AECOPD: acute exacerbations of COPD; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1s.
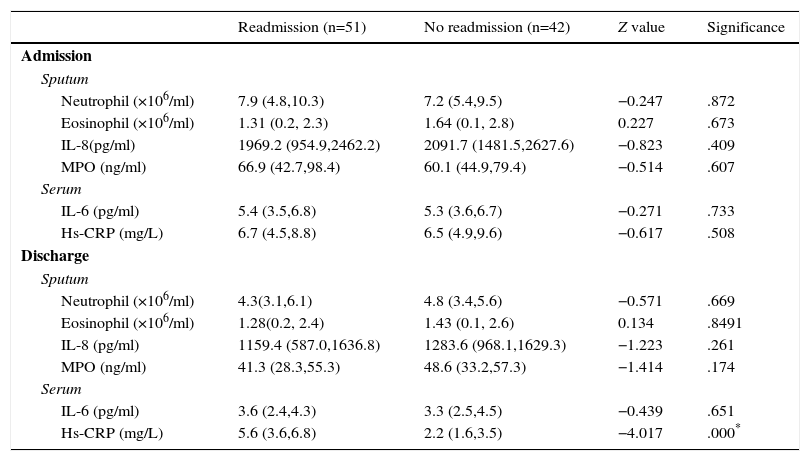
Compared with patients without readmissions, patients with readmissions had higher hs-CRP value at discharge. Neutrophils, sputum IL-8 and MPO levels, and plasma IL-6 levels at both admission and discharge were also analyzed, although no significant differences were observed (Table 2).
Role of Inflammatory Indices in Predicting Readmission for AECOPD.
| Readmission (n=51) | No readmission (n=42) | Z value | Significance | |
|---|---|---|---|---|
| Admission | ||||
| Sputum | ||||
| Neutrophil (×106/ml) | 7.9 (4.8,10.3) | 7.2 (5.4,9.5) | −0.247 | .872 |
| Eosinophil (×106/ml) | 1.31 (0.2, 2.3) | 1.64 (0.1, 2.8) | 0.227 | .673 |
| IL-8(pg/ml) | 1969.2 (954.9,2462.2) | 2091.7 (1481.5,2627.6) | −0.823 | .409 |
| MPO (ng/ml) | 66.9 (42.7,98.4) | 60.1 (44.9,79.4) | −0.514 | .607 |
| Serum | ||||
| IL-6 (pg/ml) | 5.4 (3.5,6.8) | 5.3 (3.6,6.7) | −0.271 | .733 |
| Hs-CRP (mg/L) | 6.7 (4.5,8.8) | 6.5 (4.9,9.6) | −0.617 | .508 |
| Discharge | ||||
| Sputum | ||||
| Neutrophil (×106/ml) | 4.3(3.1,6.1) | 4.8 (3.4,5.6) | −0.571 | .669 |
| Eosinophil (×106/ml) | 1.28(0.2, 2.4) | 1.43 (0.1, 2.6) | 0.134 | .8491 |
| IL-8 (pg/ml) | 1159.4 (587.0,1636.8) | 1283.6 (968.1,1629.3) | −1.223 | .261 |
| MPO (ng/ml) | 41.3 (28.3,55.3) | 48.6 (33.2,57.3) | −1.414 | .174 |
| Serum | ||||
| IL-6 (pg/ml) | 3.6 (2.4,4.3) | 3.3 (2.5,4.5) | −0.439 | .651 |
| Hs-CRP (mg/L) | 5.6 (3.6,6.8) | 2.2 (1.6,3.5) | −4.017 | .000* |
Data are presented as median (interquartile ranges) for numerical variables. Mann–Whitney U test was used for paired group analysis.
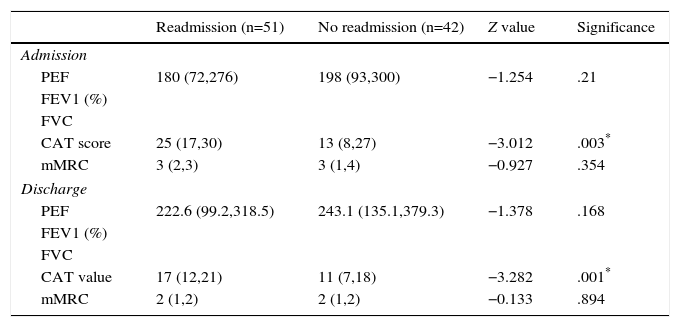
The clinical indices at admission and discharge of the two groups are listed in Table 3. Compared with patients without readmissions, patients with readmissions had higher CAT values at admission and discharge.
Role of Severity Indices of AECOPD in Predicting Readmission for AECOPD.
| Readmission (n=51) | No readmission (n=42) | Z value | Significance | |
|---|---|---|---|---|
| Admission | ||||
| PEF | 180 (72,276) | 198 (93,300) | −1.254 | .21 |
| FEV1 (%) | ||||
| FVC | ||||
| CAT score | 25 (17,30) | 13 (8,27) | −3.012 | .003* |
| mMRC | 3 (2,3) | 3 (1,4) | −0.927 | .354 |
| Discharge | ||||
| PEF | 222.6 (99.2,318.5) | 243.1 (135.1,379.3) | −1.378 | .168 |
| FEV1 (%) | ||||
| FVC | ||||
| CAT value | 17 (12,21) | 11 (7,18) | −3.282 | .001* |
| mMRC | 2 (1,2) | 2 (1,2) | −0.133 | .894 |
Univariate comparison (Tables 1–3) demonstrated that age, frequency of exacerbation in previous year ≥2, stable-status CAT value, GOLD group, diabetes, cold symptoms at presentation, hs-CRP at discharge, CAT value at admission and at day 14, met entry criteria for logistic regression. The logistic regression model (Table 4) indicated that age, hs-CRP at day 14, and CAT value at day 14 were statistically significant independent variables for predicting readmission.
Logistic Regression Data.
| Predictor | Odds ratio and 95% CI | P value |
|---|---|---|
| Age | 1.072 (1.012–1.135) | .017 |
| hs-CRP at discharge | 1.392 (1.131–1.712) | .002 |
| CAT score at discharge | 1.12 (1.031–1.217) | .007 |
Predictors with P-value of <.05 were considered to be independent risk factors for readmission for AECOPD.
We used ROC curves to identify factors with diagnostic value for readmission for AECOPD. For hs-CRP, the best cutoff value for predicting readmission for AECOPD was ≥4.165mg/L at discharge (sensitivity 74.5%, specificity 81%). For CAT, the best cutoff value for predicting readmission for AECOPD was ≥14.08 at discharge (sensitivity 66.7%, specificity 66.7%). For age, the best cutoff value for predicting readmission for AECOPD was ≥68.5 (sensitivity 51%, specificity 73.8%).
DiscussionIn this study, 54.8% patients were readmitted due to AECOPD at least once during the twelve months following the index admission, a rate similar to that reported in previous studies. Besides age, elevated levels of hs-CRP and CAT at discharge were independent risk factors for exacerbations of COPD. Exacerbations have a tendency to cluster in an individual, as demonstrated by studies of patients hospitalized with severe AECOPD. Such studies have shown that after the index exacerbation, patients are at increased risk of readmission, and one study showed that 34% of patients were readmitted with a recurrent exacerbation in the 3 months following discharge.7,15–17 Frequent readmission for COPD exacerbations have been highlighted as an independent risk factor for increased mortality.16
CRP is an acute-phase protein produced by the liver. It is elevated in most conditions associated with infection, inflammation, or tissue damage, for which it is a sensitive marker.18 C-reactive protein levels are associated with important clinical variables that help predict outcome in stable COPD patients irrespective of the degree of airflow obstruction.24 Plasma CRP levels, in the presence of a major exacerbation symptom, are useful to confirm COPD exacerbation.24 CRP levels can predict bacterial exacerbation in patients with COPD. CRP may be a useful clinical marker for antibiotic therapy in AECOPD.25
Severe AECOPD is usually resolved with treatment, but heightened CRP levels are associated with recurrent exacerbations within 50 days.12 As expected, there was an increase in sputum neutrophil inflammation during severe AECOPD.26,27 Furthermore, conventional treatment for severe AECOPD is associated with rapid reduction of airway neutrophilic inflammation.27 In this study, we did not find that airway neutrophil inflammation was related to readmission for AECOPD. This was consistent with a previous study that found plasma inflammatory markers were better predictors of recurrent exacerbations than sputum inflammatory markers.12 Age was an independent predictor of readmission, which was consistent with previous studies.28 We did not find dyspnea and peak expiratory flow (PEF) recovery on readmission. Symptoms and lung function recovery were incomplete in a significant proportion of COPD exacerbations.29 A previous study showed no significant differences in clinical indices between frequent and infrequent exacerbators.12 Another new finding in this study was that the CAT score at discharge is another independent predictor of readmission. CAT provides an objective quantification of the impact of symptoms on patients, and can be easily completed at exacerbation and during recovery. Initial studies have shown that the CAT correlates closely with health-related quality of life as measured by the St. George's Respiratory Questionnaire (SGRQ) in stable patients.30 Moreover, CAT scores increase at exacerbation and reflect exacerbation severity as determined by lung function and exacerbation length.31 A weak relationship was also found between systemic inflammatory markers and CAT scores at exacerbation. Furthermore, CAT scores in stable status are significantly elevated in patients with a history of frequent exacerbations.31
This study has important clinical implications. First, plasma CRP testing and administration of the COPD assessment test, both of which are readily available, can be performed at discharge and integrated into the routine follow-up of patients with AECOPD at no major additional cost. Second, intensive anti-inflammatory therapy should be considered for patients who have persistently higher levels of hs-CRP at discharge, due to its potential role in reducing the burden of exacerbations.
Our study has some limitations. First, the inflammatory response is complex and we studied only a limited panel of biomarkers. These, however, were used in the majority of previous studies on severe AECOPD, and are easily measured in clinical practice.29 Second, readmission may be influenced by treatment and compliance with therapy. However, all therapeutic strategies were standardized and all the patients received regular follow-up. Third, we could not include all factors that may be related to readmission for AECOPD.32 For example, we did not investigate the presence of virus with serological or molecular methods; previous studies have shown that co-infection of bacteria and virus were associated with greater impairment in terms of lung function (FEV1/FVC% and FEV1) and longer hospital stay.29 Fourth, we only included patients with severe AECOPD requiring hospitalization. This means that these results are probably not generally applicable to all populations of AECOPD patients. Fifth, the sample size should be larger in future studies.
ConclusionIn conclusion, plasma inflammatory marker was a better predictor of readmission than sputum inflammatory markers. CAT score and age were also useful to predict readmission.
Contributors- •
Zhang Jing: Experimental work and article writing.
- •
Chang Chun: Experimental work.
- •
Shen Ning: Intellectual analysis of the data.
- •
Zhu Hong: Intellectual analysis of the data.
- •
He Bei: Intellectual planning of the project.
- •
Yao Wan-zhen: Intellectual planning of the project.
This study received financial support from the Chinese Medical Association Special Fund for Research on Chronic Respiratory Diseases (Grant no. 07010440052).
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Jing Z, Chun C, Ning S, Hong Z, Bei H, Wan-zhen Y. El marcador sistémico de inflamación PCR fue mejor factor pronóstico de rehospitalización por exacerbación aguda de la enfermedad pulmonar obstructiva crónica que los marcadores de inflamación del esputo. Arch Bronconeumol. 2016;52:138–144.