To describe the clinical, functional and radiographic differences of respiratory disease caused by environmental mycobacteria in patients with and without silicosis.
MethodRetrospective, observational study in patients with nontuberculous mycobacteria isolated in the Hospital Meixoeiro (University Hospital of Vigo) microbiology laboratory between January 2007 and December 2013. Patients were grouped according to the presence or absence of silicosis and mycobacterial lung disease, using American Thoracic Society criteria.
ResultsIn 156 cases, at least one species of environmental mycobacteria had been isolated from the respiratory culture. A total of 71% were identified in men, 40 (25.6%) of whom had silicosis. Sixty patients (38.5%) met American Thoracic Society microbiological criteria: 62.5% of the silicosis group and 30.2% of the non-silicosis group. The most common species were Mycobacterium avium complex, Mycobacterium genavense and Mycobacterium chelonae. American Thoracic Society criteria for environmental mycobacterial disease were met in 34 (22.7%) patients: 14 in the silicosis group and 20 in the non-silicosis group. Treatment was administered in 24 cases, with better bacteriological eradication levels in the non-silicosis group.
ConclusionsIn our series, a history of silicosis was related with a higher incidence of environmental mycobacterial disease. The causative species in the majority of cases in our setting was Mycobacterium avium complex, followed by Mycobacterium genavense. Patients with silicosis showed lower cure rates after treatment.
Describir las diferencias clínicas, funcionales y radiográficas de la enfermedad respiratoria por micobacterias ambientales (MA) en pacientes con silicosis y sin silicosis.
MétodoEstudio observacional retrospectivo en pacientes a los que se les había aislado una micobacteria no tuberculosa en el laboratorio de Microbiología del hospital de O Meixoeiro (CHU de Vigo) desde enero 2007 hasta diciembre 2013. Se diferenció a los pacientes según presentaran o no silicosis y enfermedad pulmonar por MA utilizando los criterios de la American Thoracic Society.
ResultadosSe identificaron 156 casos con aislamiento respiratorio de al menos una especie de MA. El 71% eran varones, de los cuales 40 (25,6%) tenían silicosis. En 60 pacientes (38,5%), el 62,5% del grupo de silicosis y el 30,2% del grupo sin silicosis, se cumplían los criterios microbiológicos recomendados por la American Thoracic Society siendo las especies más comunes Mycobacterium avium complex, Mycobacterium genavense y Mycobacterium chelonae. En 34 pacientes (22,7%), 14 del grupo de silicosis y 20 del grupo sin silicosis, se cumplían los criterios de la American Thoracic Society de enfermedad pulmonar por MA. Se realizó tratamiento en 24 casos, con mayores niveles de erradicación bacteriológica en el grupo sin silicosis.
ConclusionesEn nuestros pacientes el antecedente de silicosis se relacionó con mayor incidencia de enfermedad por MA. La especie causante de la mayor parte de los casos de de enfermedad en nuestro medio por MA es Mycobacterium avium complex, seguido de Mycobacterium genavense. Los pacientes con silicosis presentaron menores niveles de curación tras el tratamiento.
Nontuberculous mycobacteria (NTM), also known as environmental mycobacteria, are ubiquitous microorganisms that can inhabit body surfaces and secretions, even in the respiratory tract, without causing disease. For this reason they were considered purely as colonizers until the latter half of the last century. The geographical prevalence of the different mycobacterial species varies widely,1 and accurate identification is essential, due to differences in their clinical relevance. In countries with low rates of pulmonary tuberculosis (PTB), such as the United States, the incidence of NTM (5 cases per 100000 and up to 15 per 100000 in individuals over 50 years of age) is higher than that of PTB (4 per 100000).2 Known risk factors for NTM lung disease include cystic fibrosis and other diseases presenting with bronchiectasis, and immunosuppressive states, such as those associated with biological therapy (anti-TNF, rituximab), organ transplantation, and autoimmune deficiency syndrome, although an increasing number of cases of NTM are now detected in patients with no underlying disease.2 A risk factor which has received less attention is NTM lung disease in silicosis. Although the relationship between silicosis and PTB is well characterized,3 the role of silicosis as a risk factor for NTM lung disease is less well defined.4 NTM infection most commonly presents as chronic lung disease. Two radiographic patterns are observed: fibrocavitation in the upper lobes of patients with underlying respiratory disease, such as chronic obstructive pulmonary disease; and bronchiectasis in the right middle lobe or the lingula.5 For a diagnosis of NTM lung disease, certain clinical, bacteriological and radiological criteria must be met.6 Radiological criteria in particular are difficult to determine in cases with silicosis, as the pre-existing interstitial-nodular disease makes it difficult to determine if the NTM isolated from the sputum constitutes mycobacterial disease or simply colonization. Accordingly, a detailed evaluation of symptoms and radiological findings is essential. Although the pathogenic potential of NTM is being increasingly recognized, the clinical relevance of NTM has not been determined. The aim of this study was to identify the different species isolated in NTM-infected patients with and without silicosis in our setting, and to describe the clinical characteristics, predisposing factors, and the progress of patients in both groups.
Materials and MethodsWe conducted a retrospective observational study of patients with confirmed NTM infection, determined by the Microbiology Laboratory of the Hospital O Meixoeiro (University Hospital Complex, Vigo, Spain) between January 2007 and December 2013. Species were identified, isolation rates were determined, and 2 groups were categorized: those who met diagnostic criteria for silicosis, and those who did not.7 Age, sex, underlying respiratory disease, comorbidities [diabetes, solid tumor disease or hematological malignancies, human immunodeficiency virus (HIV), transplantation], and use of corticosteroids and other immunosuppressive drugs were collected. History of exposure to silica dust and radiological findings consistent with silicosis were recorded. Airflow obstruction was defined as FEV1/FVC ratio of less than 0.7.8 Symptoms, chest X-ray and computed tomography (CT) findings were evaluated, if available, particularly in the presence of cavitation.
A diagnosis of NTM lung disease was established on the basis of clinical, radiological and bacteriological data according to the 3 categories recommended by the American Thoracic Society: definitive disease, clinically significant infection (or possible disease, if microbiological criteria were met), and colonization, in the case of positive mycobacterial culture only.6,9 Definitive disease was defined as: (a) consistent symptoms (cough, fever, weight loss, hemoptysis or dyspnea) not attributable to other diseases; (b) characteristic lesions on chest X-ray (infiltrates, nodules or cavitation) or on high resolution computed tomography (multiple nodules or multifocal bronchiectasis), and (c) 2 positive sputum cultures on 2 separate occasions, or positive bronchoalveolar lavage culture, or any positive culture of a bronchopulmonary biopsy, or else acid-fast bacilli visualized on any positive sputum culture. Cases with 2 or more positive microbiological cultures were considered clinically significant infection, and a single positive microbiological culture obtained in the absence of other qualifying criteria was defined as colonization. These microbiological criteria were selected in view of their proven utility as good predictors of disease.9–11
Bacteria were identified using molecular and radiometric methods. Response to treatment was assessed from clinical, radiological and microbiological progress. Bacteriological conversion was defined as three separate consecutive negative cultures, at least 3 weeks after the initial diagnosis of the disease.
A descriptive data analysis was performed: mycobacteria species, underlying disease and silicosis and other patient-related variables were presented as frequencies and percentages. Univariate analyses were performed using the chi-squared test to determine the association between silicosis and baseline patient characteristics, type of mycobacteria, disease prevalence, infection and colonization, treatment, and symptoms. The relationship between age and silicosis was analyzed using the Student's t-test for independent samples. Analyses were performed using the Statistical Package for Social Sciences version 15.0 (SPSS, Chicago, IL, USA).
ResultsWe reviewed 156 clinical records which contained at least 1 record of a positive NTM microbiological culture in respiratory samples and clinical and radiological data. Most samples were sputum, except for 20 cases of bronchial aspirate or bronchoalveolar lavage (in 10 cases, the culture was from these samples only). In 3 patients, the same NTM was isolated in both a cervical node aspirate and a respiratory sample – in 2 cases Mycobacterium avium complex (M. avium complex) and in 1 case Mycobacterium genavense (M. genavense). Of the 156 patients, 111 (71.2%) were men and 45 were women (28.8%), with a mean age of 62±17 years (range: 26–97). A total of 97 patients (68.2%) had some form of underlying lung disease: silicosis (25.6%), chronic obstructive pulmonary disease (14.1%), previous or active tuberculosis (11%), bronchiectasis (10.3%) and other respiratory diseases (11.5%). Other concomitant diseases included solid tumors (7.6%), HIV infection (3.8%), heart disease (3.8%) and liver disease (3.2%). Twenty-one patients (13.4%) had no underlying disease. The number of mycobacteria identified in our hospital was 7 in 2007, 14 in 2008, 32 in 2009, 41 in 2010, 25 in 2010, 26 in 2011 and 11 in 2013.
The most frequently mycobacteria isolated (Fig. 1) were M. avium complex (100), Mycobacterium gordonae (M. gordonae) (19) and Mycobacterium chelonae (M. chelonae) (13); 23 mycobacteria were unidentified (M. sp.). Fig. 1 also shows the distribution of NTM isolated in the 60 clinically significant cases (≥2 positive cultures). The species most commonly isolated in this group of patients was also M. avium complex, present in 76.7% of cases.
A diagnosis of silicosis was confirmed in 40 (25.6%) patients, compared to 116 (74.4%) without silicosis. All silicosis patients were men, with a mean age of 56.2 years [standard deviation (SD) 13.1; range: 36–85]. The patients without silicosis were significantly older (63.9 years) (SD 17.2; range: 26–94); 71 (61.2%) were men.
The principal characteristics of both groups are shown in Table 1. The most notable features are the higher rates of smoking, airflow obstruction, and use of inhaled corticosteroids in the silicosis group. Silicosis patients presented airflow obstruction (60.5%) more often than patients without (35.1%), and all (100%) of patients with silicosis and NTM disease had airflow obstruction. Diabetes mellitus, on the other hand, was more common in the group without silicosis. A total of 22 patients (7 with silicosis, and 15 without) had a history of previous PTB; 6 (3 in each group) had active tuberculosis, 5 pulmonary and 1 lymph node tuberculosis, all of whom had concomitant NTB in a single sputum sample, interpreted as NTM colonization. In patients without previous disease, the positive culture was isolated and interpreted as colonization.
Characteristics of Patients In Whom Nontuberculous Mycobacteria Were Isolated.
| Characteristics | SilicosisN=40 | No silicosisN=116 | P-value |
|---|---|---|---|
| Age | 56.2±13.1 | 63.9±17.2 | .004 (*) |
| Sex | |||
| Women | 0 (0%) | 45 (38.8%) | <.001 (*) |
| Men | 40 (100%) | 71 (61.2%) | |
| Previous PTB | 7 (17.5%) | 15 (12.9%) | .650 |
| Active PTB | 3 (7.5%) | 3 (2.6%) | .359 |
| Smoking habit | |||
| Non-smoker | 14 (35%) | 63 (59.4%) | |
| Former smoker | 20 (50%) | 26 (24.5%) | .010 (*) |
| Active smoker | 6 (15%) | 17 (16%) | |
| Diabetes | 1 (2.5%) | 21 (18.1%) | .014 (*) |
| FEV1/FVC<70 | 23 (60.5%/38) | 13 (35.1%/37) | .028 (*) |
| Steroid use | |||
| Oral | 8 (20%) | 19 (16.4%) | .602 |
| Inhaled | 24 (60%) | 39 (33.6%) | .003 (*) |
| Immunosuppressants | 0 (0%) | 3 (2.6%) | .570 |
Criteria recommended by the American Thoracic Society for the diagnosis of NTM pulmonary disease were met in 34 patients, 21% of all cases, and in 56% of those who met the microbiological criteria (14 with silicosis and 20 without).
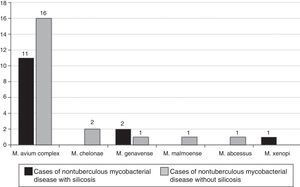
The NTM strains isolated in the 14 patients with silicosis who met the microbiological criteria recommended by the American Thoracic Society were (Fig. 2): M. avium complex (n=11; 71.4%), M. genavense (n=2) and Mycobacterium xenopi (M. xenopi) (n=1), and the NTM strains isolated in the 20 non-silicosis patients were: M. avium complex (n=19; 95.0%), M. gordonae (n=1), M. genavense (n=1), Mycobacterium fortuitum (M. fortuitum) (n=1), Mycobacterium abscessus (M. abscessus) (n=1), Mycobacterium malmoense (n=1), M. chelonae (n=1) and Mycobacterium celatum (n=1); 2 mycobacteria were not identified.
The predominant symptom in silicosis patients was dyspnea, detected in 30 (75%); in patients without silicosis, the predominant symptom was cough, seen in 41 (35.3%). Cavitation was observed in 29% of patients with a diagnosis of NTM disease (57% in the silicosis group, and 10% in the non-silicosis group). The most common CT findings in silicosis were nodules and conglomerates (52.5%), and in non-silicosis patients, bronchiectasis (60.0%) (Table 2).
Clinical, Radiological and Microbiological Characteristics. Progress of Treated Patients.
| Characteristics | SilicosisN=40 | No silicosisN=116 | P-value |
|---|---|---|---|
| Symptoms | |||
| Asymptomatic | 2 (5%) | 25 (21.6%) | .032 (*) |
| Dyspnea | 30 (75%) | 34 (29.3%) | <.001 (*) |
| Cough and expectoration | 7 (17.5%) | 41 (35.3%) | .035 (*) |
| Cavitation | 9 (22.5%) | 9 (7.8%) | 0.012 (*) |
| CT (40/84) | |||
| Nodules | 18 (45%) | 12 (14.3%) | <.001 (*) |
| Conglomerates | 21 (52.5%) | 0 (0%) | <.001 (*) |
| Bronchiectasis | 0 (0%) | 21 (25%) | .001 (*) |
| Sputum smear (+) | 3 (7.5%) | 7 (6%) | .744 |
| Bronchial aspirate | 4 (10%) (4 sputum +) | 15 (12.9%) (11 sputum +) | .835 |
| Cases | |||
| Colonization | 15 (37.5%) | 81 (69.8%) | |
| Infection | 11 (27.5%) | 15 (12.9%) | .001 (*) |
| Disease | 14 (35%) | 20 (17.2%) | |
| Treatment | 9 (22.5%) | 14 (12.1%) | .109 |
| Eradication/cure | 3 (33.3%) | 8 (57.1%) | .531 |
| Under treatment | 5 (55.5%) | 5 (35.7%) | .612 |
| Death | 1 (11.1%) | 1 (7.1%) | .668 |
Of the 34 patients with disease criteria, 23 (67%) were treated (9 silicosis and 14 non-silicosis) (Table 2). Cultures became negative in 3 (33%) of the 9 cases which received specific treatment (2 M. avium complex and 1 M. xenopi), but treatment failed in 5 (3 M. avium complex and 2 M. genavense). Bacteriological eradication was achieved in 8 (57%) of the non-silicosis group, all of whom had M. avium complex, but treatment failure was also observed in 5 cases (4 M. avium complex and 1 M. abscessus). One patient in each group died: in the silicosis group, early death was associated with pneumothorax; and in the non-silicosis group, death was due to drug-induced hepatitis. In the non-silicosis group, a second case of toxicity in the form of cholestatic hepatitis was observed, but medications could be reintroduced without complications. Two patients (1 with silicosis and 1 with chronic obstructive pulmonary disease) were successfully treated for 12 months before lung transplantation. At the end of the study period, the survival rate was 76.4%, with no differences between the silicosis and non-silicosis groups, nor in colonization, infection and disease rates in the 113 patients in whom NTM infection was confirmed.
DiscussionSilicosis is associated with the development of NTM infection and disease, and is the most common predisposing factor for NTM infection in our environment. Prevalence is clearly higher than reported in clinical epidemiological NTM studies performed in Spain, which found silicosis responsible for NTM disease in 0.9%−2.3% of cases.12,13
Recent studies14,15 underline the importance of NTM in silicosis patients. A systematic search by Morita et al.16 reported a positive culture rate of 39% for pathogenic and non-pathogenic NTB species in these patients. The high prevalence of silicosis in Spain − associated with the country's sizeable granite industry (quarries and workshops)17 – may explain the high percentage of silicosis identified as a cause of NTB disease.
NTM lung disease tends to be more often associated with silicosis than with PTB. Publications which included all notifiable cases of PTB in our autonomous community during the same period as our study show a prevalence of silicosis of 0.5%18; this is far lower than the 41% prevalence of silicosis we found among our series of patients with NTM lung disease. Corbett et al.14 similarly reported a higher incidence of pneumoconiosis among NTM patients than among those with M. tuberculosis infection. The rising rate of positive NTM cultures does not seem to be exclusively associated with improvements in laboratory methods: other factors may be contributing, such as greater environmental exposure to NTB (exposure to NTM is increasing due to factors such as climate change, earthworks, aerosolized water particles, etc.), underlying host factors (bronchiectasis, chronic aspiration, chronic obstructive pulmonary disease, advancing age, etc.) or innate host characteristics associated with NTM disease (women with slender body habitus, scoliosis, pectus excavatum, and marfanoid habitus).2
In our hospital, a high percentage of patients (61.5%) had only 1 positive culture and were considered colonized. This rate was relatively high compared to that of other authors: Andréjak et al.9 reported 55%, Winthrop et al.10 reported 42% in their review, and Braun et al.19 found only 11.2%. We may have found a higher rate of “colonized” patients because no evidence of subsequent evaluations during the study period was found in the clinical records of many of our patients with 1 positive culture.
The most common colonizing species were M. avium complex, M. gordonae and M. chelonae. The first 2, M. avium complex and M. gordonae, were also reported as the most common in some series conducted in our setting,1 in contrast with other series in which M. gordonae, M. xenopi, M. fortuitum, Mycobacterium terrae, Mycobacterium simiae, and others were predominant. This may be explained by regional differences in the distribution of species.20,21
The number of cases of NTM disease (14 with silicosis and 20 without) found in our series, representing 21% of all positive respiratory cultures, was below that of some reports in the literature, in which 33%–50% of cultures represented definitive disease.9,10 Similar results to ours were found by Koh et al.,20 who reported 17%, and Andréjak et al.,9 who reported 26%, whereas our rates were higher than those of Braun et al,19 who reported 9.8%. A greater number of subjects, now outside the study period, may have met the disease criteria.
The species most often isolated in patients who met NTM disease criteria, both in silicosis and non-silicosis cases, was M. avium complex. In contrast to other studies (some performed in Spain13,14) which reported Mycobacterium kansasii (M. kansasii) as the most prevalent NTM, this species was almost absent from our series.
Another unusual finding in our series was that the second most frequently isolated species associated with disease was M. genavense, rarely reported in the literature and absent from other publications.9,10 This species is associated with HIV (before the highly-active antiretroviral therapy era) and with severe immunosuppression, situations in which it generally appears as disseminated disease.22 Our patients with M. genavense disease included 2 silicosis patients, 1 with exclusively pulmonary involvement, who was receiving high-dose corticosteroids (prednisone 30mg/day), and the other with cervical lymphadenitis with no known immunosuppression. The other patient with M. genavense disease had HIV infection, with pulmonary involvement and cervical lymphadenitis.
In our series, 57% of the patients who met the microbiological criteria also met the criteria for disease. This figure was lower than the 86% of Winthrop et al.10 We are probably underestimating the real prevalence of NTM lung disease by wrongly classifying as indeterminate disease cases of positive NTM culture in some patients who met the microbiological criteria, but for whom either no CT results were available or whose underlying disease (silicosis or PTB) made it difficult to determine the presence of signs associated with NTM disease.
A total of 63 (40%) of our patients were receiving inhaled corticosteroids (60% in the silicosis group and 33% in the non-silicosis group). This difference was significant in favor of silicosis, underlining the risk of NTM disease associated with the use of inhaled steroids pointed out by Andréjak et al.23 In contrast to these authors, however, we found no cases of NTM associated with other immunosuppressants such as TNF-α antagonists,24 despite their extensive use, or with cystic fibrosis.25
Cavitation was observed in 25% of patients with NTM disease. This figure is similar to other recent series,9 particularly in silicosis, but far from the 76% rate of cavitating infiltrates in HIV-negative patients reported by Martínez-Moragón et al.12
Our cure rates of 30% and 57% (bacteriological eradication) in silicosis and non-silicosis patients, respectively, are lower than other reports from Spain which describe a generally favorable clinical progress among treated patients, with bacteriological eradication in up to 75% of cases,12 but similar to the 57.7% reported by Kobashi et al.26 This lower treatment efficacy may be attributed to several causes mentioned in the literature, such as the relative frequency of cavitation in silicosis patients, previous treatment for M. avium complex lung disease, and a history of chronic obstructive pulmonary disease or bronchiectasis.6,27 However, in our study, this result may be due to the low number of patients treated. Deaths in our series were associated with the underlying disease status of the patients, unlike 2 of the 11 treated patients from the series of Fujita et al.28 who died due to respiratory infection attributed to NTM infection.
Our study may have some limitations, primarily its retrospective nature. This prevented us from further investigating any unclear aspects in the patients’ clinical records, which may have led to a more accurate classification of the cases. Moreover, if the treating physicians had conducted a more comprehensive diagnostic process at the time, some patients may have been assigned to a different diagnostic group.
In conclusion, a history of silicosis was related in our setting with a higher incidence of NTM disease. The patient's underlying disease did not appear to modify the types of species identified, the most common being M. avium complex in both silicosis patients and non-silicosis patients. In our study, we found a rate of M. genavense that is not echoed in other reviews of NTM. It would be of interest to study the results of cultures taken in coming years, to see if the relevance of this strain is confirmed in our setting. Finally, our cure rates appear to be low. These results suggest that we need to optimize management of these patients and consolidate the diagnostic suspicion of NTM disease in order to improve diagnostic accuracy and cure rates.
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Blanco Pérez JJ, Pérez González A, Morano Amado LE, Guerra Vales JL, Vázquez Gallardo R, Salgado Barreira Á, et al. Significado clínico de las micobacterias ambientales aisladas en muestras respiratorias en pacientes con silicosis y sin silicosis. Arch Bronconeumol. 2016;52:145–150.