Venous thromboembolic disease (VTD) is a health problem of the first order, with an incidence of deep vein thrombosis (DVT) of 145 per 100,000 inhabitants and pulmonary thromboembolism (PTE) of 65.8 per 100,000 inhabitants.1,2 Three factors are responsible for venous thrombosis: blood stasis, vascular damage, and a hypercoagulable state, that can be congenital or acquired.3 The latter may be due to malignant, autoimmune, or infectious diseases. The use of complementary examinations for the detection of occult cancer in patients with VTD is controversial, so tools are being developed to improve the identification of these patients.4 The manifestations of hypercoagulability associated with cancer include the common, conventional conditions, such as pulmonary thromboembolism (PTE), deep vein thrombosis (DVT), and migratory thrombophlebitis, more uncommon entities, such as arterial thrombosis, and the rare, difficult-to-diagnose entities, such as non-bacterial thrombotic endocarditis (NBTE).5
We retrieved very few references from the literature on the subject (Medline and Pubmed search engines, keywords “endocarditis, non-infective” and “pulmonary embolism”). Only 3 cases have been reported in which both entities coexist, and all of them were associated with underlying cancers of the lung,6 ovaries,7 and pancreas.8
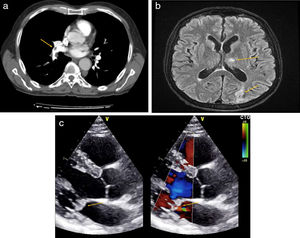
We report the case of a 64-year-old man, former smoker of 20 pack-years who gave up 5 years previously, with a personal history of vitiligo, receiving treatment with omeprazole 20mg. He consulted due to sudden onset of dyspnea, pleuritic-type chest pain, and syncope. Physical exploration revealed O2 saturation 87% and tachycardia 134 beats per minute. Most significant findings included a systolic murmur i/vi in the mitral valve, non-tender hepatomegaly measuring 3cm on the costal margin, and a solid mass in the right lower limb. Complete blood count (CBC) showed microcytic anemia (hemoglobin 9.9g/dl), with ferritin 1728ng/ml (normal values [NV] 30–400ng/ml). Biochemistry was significant for lactate dehydrogenase 814U/l (NV: 125–250U/l), glutamic pyruvic transaminase 71U/l (NV: 2–33U/l), C-reactive protein 193mg/l (VN 0–5mg/l), and D-dimer >20,000ng/ml. Computed tomography angiography (CTA) showed filling defects in the arteries of the lower right and left lobes compatible with PTE (Fig. 1a). Lower limb venous Doppler showed a femoro-popliteal DVT in the right lower limb. Based on these findings, the patient was admitted and began treatment with enoxaparin adjusted for weight every 12h.
(a) CT angiography of pulmonary arteries. Filling defect in arteries of the right and left lower lobes consistent with PTE, arrow. (b) Brain MRI in T2. Ischemic impacts in cerebellar hemispheres and in the occipital region, arrows. (c) Echocardiogram in the long parasternal axis. Severe mitral insufficiency and endocardial masses on mitral valve, indicated with arrows.
The patient reported a weight loss of 6kg in 2 months, asthenia, and fever. Given this constitutional syndrome, an abdominal CT scan was performed, which revealed a mass measuring 38×37mm in the body of the pancreas and multiple liver masses consistent with pancreatic cancer with liver metastasis. Fine-needle aspiration with endoscopic ultrasonography confirmed the presence of pancreatic adenocarcinoma.
Seventy-two hours later, the patient presented loss of strength in the right upper limb and reduced level of consciousness. The neurological examination revealed a loss of strength in the proximal right arm, accompanied by an increased base of support and positive Romberg test. CT of the brain revealed hypodense lesions without contrast uptake in both cerebellar hemispheres and the right occipital lobe. Brain magnetic resonance imaging (MRI) confirmed the presence of ischemic lesions (Fig. 1b). In view of suspected embolic syndrome, we performed a transthoracic echocardiogram that showed severe mitral insufficiency and endocardial masses on the mitral valve, while the presence of patent foramen ovale was ruled out (Fig. 1c). Blood cultures were repeatedly negative, as were serologies for Coxiella spp., Brucella spp., Bartonella spp., and Legionella spp.
With a diagnosis of VTD, NBTE, and stroke of embolic origin caused by a hypercoagulable state due to stage IV pancreatic adenocarcinoma (T2N1M1), the treating physicians decided to initiate treatment with tinzaparin 14,000IU every 24h and palliative chemotherapy.
NBTE, formerly known as marantic endocarditis, is a rare clinical entity that has devastating consequences.5 It may appear concurrently with VTD as an expression of a procoagulant state or be the primary source of the embolic disease.
Ziegler9 first described NBTE in 1888 using the term “thromboendocarditis”, but it was not until 1954 that Angrist and Marquiss highlighted the strong association of systemic emboli with this entity.10 It is characterized by the presence of fibrin vegetations on the heart valves in the absence of bacterial infection.5,11 The valves typically affected are the mitral and aortic valves, although the involvement of right heart valves has also been less commonly reported.
The incidence on autopsy ranges between 0.3% and 9.3%.11 This contrasts with the low incidence of diagnosis in clinical practice,8,12 and demonstrates the need for a high clinical suspicion. Like VTD, it is associated with a wide variety of procoagulant states, such as autoimmune diseases, infections, and cancer, especially of the pancreas and lung.13
The pathogenesis of NBTE involves the interaction between macrophages and malignant cells, which releases cytokines that damage the endothelium and promote the aggregation and deposition of platelets and thrombus formation. Overactivation of the coagulation cascade provokes a hypercoagulable state, which fosters the development of thrombi. This leads to the growth of sterile vegetations composed of fibrin and blood platelets.14
Clinically, it manifests with systemic embolisms and, more rarely, valvular dysfunction. Heart murmurs are uncommon, and tend to be systolic if present, complicating the suspected diagnosis. Diagnosis is clinical, accompanied by the demonstration of vegetations on echocardiography.
The main sites of embolization are the spleen, kidney, and limbs, while embolic events in the coronary arteries and the central nervous system produce greater morbidity and mortality.9,10 Although it tends to occur in the left valves, pulmonary circulation is frequently affected by the embolic phenomenon.15
Treatment is based on control of the underlying disease and anticoagulation with heparin or low molecular weight heparin; vitamin K antagonists should be avoided. Valvular surgery should be considered an option in selected patients.8
We believe that this case of synchronous presentation of NBTE and PTE illustrates the need for clinical suspicion in patients with VTD and a clinical picture of systemic embolism, after other more common entities such as patent foramen ovale have been ruled out. NBTE is a rare entity that is difficult to diagnose, but one that has great prognostic relevance for the patient.
Please cite this article as: Martín Guerra JM, Asenjo MM, Dueñas Gutiérrez CJ, Gil González I. Consecuencias cardiopulmonares sincrónicas del estado de hipercoagulabilidad asociado al cáncer. Arch Bronconeumol. 2019;55:330–332.