Thoracic involvement has been described in the course of multiple myeloma (MM), in the form of bone lesions, extraosseous plasmacytomas, pulmonary infiltration and, exceptionally, pleural effusion (PE). Myelomatous pleural effusion (MPE) occurs in only 1% of cases of PE in patients with MM, and is associated with a poor prognosis (median survival of 1.5–3 months after appearance). We report a patient with MM with extramedullary thoracic involvement who developed secondary MPE.1,2
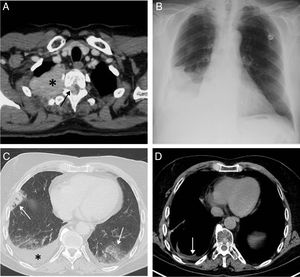
This was a 67-year-old man with a history of quiescent MM IgA kappa diagnosed in 1998 who attended the emergency room of our hospital in November 2017 due to fever, general malaise, asthenia, and cough. The patient had presented several plasmacytomas in the right chest wall (Fig. 1A) and the spinal canal in 2012 and 2014, but without PE, and was treated with different therapeutic options (chemotherapy, autologous hematopoietic stem cell transplantation, local radiation therapy), resulting in complete resolution of the lesions. In 2015, he presented a new extramedullary relapse in the form of a paravertebral mass in the right hemithorax, which was treated with chemotherapy. Given the patient's clinical picture and radiographic findings (not present in previous studies) of right PE observed in the emergency department (Fig. 1B), we decided to admit him and perform a positron emission tomography/computed tomography (PET/CT). PET/CT showed bilateral hypermetabolic pneumonic pulmonary opacities and confirmed a moderate amount of right PE (Fig. 1C). Serosanguineous pleural fluid with the following characteristics was obtained: pH 7.43, glucose 104mg/dl (serum glucose 91mg/dl), lactate 3.2mmol/l, pleural fluid protein/serum protein ratio: 0.70, pleural fluid LDH/serum LDH ratio: 2.37, LDH pleural fluid: 524, hematocrit <15%, lymphocytes 28.7%, neutrophils 0.0% (criteria for lymphocytic exudate) and negative microbiological tests, thus ruling out PE due to infection. Flow cytometry of pleural fluid detected 60% large neoplastic plasma cells (plasmablasts), CD 138+ and CD 56−, which confirmed the malignant nature of the PE (myelomatous). Jugal mucosa biopsy ruled out the presence of amyloid deposits. The patient was initially treated with antibiotics (piperacillin–tazobactam) and subsequently underwent right pleural drainage followed by chemical pleurodesis with talc, local radiotherapy and chemotherapy (pomalidomide–dexamethasone–cyclophosphamide), presenting excellent clinical and radiological progress (Fig. 1D).
(A) Axial CT scan of the chest (mediastinum window) performed in 2012 that identifies a solid mass in the upper right hemithorax (asterisk) infiltrating the chest wall and penetrating the spinal canal through the right T2–T3 intervertebral foramen (arrow). (B) Posteroanterior chest X-ray performed in November 2017 showing right pleural effusion for the first time in this patient. (C) Chest axial image of a PET/CT scan performed in November 2017 which identifies bilateral opacities of pneumonic aspect (arrows) and right pleural effusion (asterisk). (D) Thoracic axial image of another PET/CT scan performed in March 2018 which shows the disappearance of pneumonic opacities and the presence of minimal pleural effusion.
During the course of MM, 15%–30% of patients may develop extramedullary involvement.1 The pleural cavity is an unusual site for MM recurrence; in fact, MPE occurs in only in 1% of cases of PE in patients with MM. In a recently published series, MPE represented only 0.6% of malignant PEs.2,3 Diagnostic criteria for MPE are: (1) presence of atypical plasma cells in pleural fluid (neoplastic plasma cells or a monoclonal component); (2) pleural biopsy consistent with neoplastic plasma cells, or (3) demonstration of monoclonal proteins in the pleural fluid by electrophoresis. In doubtful cases, flow cytometry helps establish the immunophenotype of neoplastic plasma cells compared to that of reactive cells. When a patient with MM develops PE, it is important to rule out common etiologies, such as paraneumonic PE, heart failure, kidney failure, and amyloidosis. The latter may cause PE due to cardiac (heart failure), renal (nephrotic syndrome), liver (ascites), or pleuropulmonary involvement.4 MPE can be caused by an abnormal proliferation of plasma cells from an extramedullary plasmacytoma of the chest wall, invasion from an adjacent bone lesion, or direct invasion of the pleura by myeloma.5 While various treatments are available for MPE (chemotherapy, therapeutic thoracentesis, chest drain, or pleurodesis), there is no consensus about how we should manage these patients. Extramedullary involvement is associated with an adverse prognosis, especially when it is recurrent MM. Pleural infiltration is usually fatal, with a median survival of 1.5–3 months. Therefore, more aggressive chemotherapy regimens can be indicated in MM with involvement of the pleural cavities. Our patient responded well to multimodal treatment consisting of a combination of radiation therapy, chemotherapy, and chemical pleurodesis, achieving clinical remission that lasted 6 months after diagnosis of the MPE.
This case reminds us that we must investigate all the causes of PE in patients with a history of MM and that, although the incidence of MPE is low, it must be taken into account as a diagnostic possibility. Its grim prognosis and aggressive natural course require us to make a quick and appropriate diagnosis in order to start treatment as soon as possible.
Please cite this article as: Velasco-Álvarez D, Gorospe-Sarasúa L, Fra-Fernández S, Blanchard MJ. Mieloma múltiple extramedular con afectación pleural: una rara entidad clínica. Arch Bronconeumol. 2019;55:332–333.