REDAAT, the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency, was set up in order to improve knowledge of this disease. This study is an evaluation of the registry and an analysis of its patient population.
MethodsThe registry has a database hosted on the website www.redaat.es. It collects clinical and functional data on patients with PiSZ, ZZ phenotypes and other rare variants.
ResultsThanks to the collaboration of 124 physicians, the registry currently contains information on 511 individuals from 103 healthcare centers. Of these 511, 348 (74.2%) are Pi*ZZ homozygotes, and 100 (19.5%) are Pi*SZ heterozygotes. More cases are seen in tertiary level hospitals. A total of 81% of the cases have respiratory disease, and a lower proportion of AATD cases were detected by family screening or liver disease. Follow-up data are available for 45% of the cases, and 35% received alpha-1 antitripsin replacement therapy.
ConclusionsThe REDAAT registry is a useful tool for obtaining quality information about this minority disease in routine clinical practice conditions, although it is difficult to obtain follow-up data, and the representativeness of the sample included cannot be determined.
El Registro español de pacientes con déficit de alfa-1 antitripsina (REDAAT) se formó con el objetivo de mejorar el conocimiento sobre del DAAT. En este trabajo se evalúa el registro y se analiza la población de pacientes incluida en él.
MétodosDispone de una base de datos alojada en la Web: www.redaat.es. Su base de datos recoge información clínica y funcional de individuos portadores de los fenotipos PiSZ, ZZ y variantes raras.
ResultadosEn la actualidad reúne información sobre 511 individuos procedentes de 103 centros sanitarios, gracias a la colaboración de 124 médicos. De ellos, 348 (74,2%) son homocigotos Pi*ZZ y 100 (19,5%) heterocigotos Pi*SZ. Existe una mayor concentración de casos en hospitales universitarios de tercer nivel. El 81% de los casos tiene enfermedad pulmonar y en menor proporción el DAAT se detectó por cribado familiar o enfermedad hepática. Se dispone de datos de seguimiento en el 45% de los casos, y un 35% recibieron tratamiento sustitutivo con alfa-1 antitripsina.
ConclusionesEl REDAAT es una herramienta útil para obtener información de calidad sobre esta enfermedad minoritaria en condiciones de práctica clínica habitual, aunque obtener datos de seguimiento es difícil y no es posible conocer la representatividad de la muestra incluida.
REDAAT, the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency, was set up in 1993 after the diagnosis of the first cases in Barcelona and Asturias. It functioned primarily as a working group within the area of respiratory failure and sleep disorders, and the scope was later extended to include the area of chronic obstructive pulmonary disease (COPD) of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).1–4
Since it was founded, the objectives of REDAAT have been to broaden the DAAT knowledge base by stimulating research and facilitating diagnosis.
The REDAAT participates in the Alpha-1 Antitrypsin International Registry (AIR) that was founded in 1996 following the recommendations of the World Health Organization (WHO).5 It also forms part of the Spanish Network of Rare Disease Registries by participating in the National Registry of Rare Diseases run by the Rare Diseases Research Institute (ISCIII Institute of Health).6,7
This study evaluated the registry as a tool for the systematic collection of data under standard clinical practice conditions, and examined the profiles of participating doctors and the demographic, phenotypic, clinical and functional characteristics of patients included during the 20 years since it was initiated.
MethodOrganization of the RegistryREDAAT consists of an advisory committee, formed by 10 pulmonologists, 3 pediatricians, and 3 basic investigators, reference laboratory personnel and information technology support staff. The main technical resource is the website: www.redaat.es, a domain of the Spanish Pulmón-Respira Foundation. The website has a public access area that contains general information and a restricted access area for health professionals that includes the patient data collection sheet, real time data on registered cases, and general characteristics of the population included. It also provides information on diagnosis and replacement therapy.7
Inclusion of cases in the REDAAT registry has gone through 3 phases: initially, from registry set-up until 2001, the data collection sheet for each case was submitted on paper to the coordination center, which at that time was located in the Hospital Universitari Vall d’Hebron de Barcelona. The second phase spanned the period between 2001 and 2005, during which the online registry was launched and paper records were gradually phased out. The third phase of exclusively web-based data management began in 2006 and continues to this day.8
Database StructureThe REDAAT database is hosted on the website www.redaat.es, and can also be accessed via the following websites: www.separ.es, https://spainrdr.isciii.es, and the AIR website, www.antitrypsindeficiency.org. The data collection questionnaire was adapted from the AIR template in HTML and connects to an Oracle database.
Each individual register consists of a 3-digit number and the initials of the patient and the treating physician. Only the latter has access to the personal data identifying the registered individual. Patients must sign informed consent forms before their data are included.
In addition to the initial registration of the case, 6-monthly follow-up sheets are available in REDAAT for collecting data on the progress of patients until the case is closed, which occurs when the patient dies or undergoes lung transplantation.
Study PopulationThe study sample includes all individuals registered in REDAAT up to January 1, 2014. Criteria for inclusion in the registry are as follows: individuals with severe AAT deficiency, carriers of Pi*ZZ and Pi*SZ phenotypes or other rare deficiency variants. Individuals with intermediate deficiency and Pi*MZ, Pi*MS and Pi*SS phenotypes were excluded.
Statistical AnalysisFirst, we reviewed each variable and the percentage of completion as a measure of data collection quality. The number of patients included in REDAAT was then compared with data on the estimated prevalence of the deficiency in Spain, to determine the magnitude of underdiagnosis.
A descriptive study was made of the characteristics of individuals included in the database, and for qualitative variables, frequency and percentage of valid data were determined. For quantitative variables, central tendency measures (mean and median), position measures (quartiles) and dispersion measures (standard deviation) were used.
Comparison by “index case” and “non-index case” was made by classifying the cases as follows: the “index case” was considered the case in which the presence of lung or liver disease led to the AATD diagnosis, while a “non-index” case was one in which the diagnosis was reached by some type of screening. With regard to treatment, replacement therapy was deemed to have been administered to any patients who at any time after registration had reported starting replacement therapy, irrespective of dose or duration of the treatment.
Comparisons between both groups were performed using the Chi-squared test for qualitative variables, the Fisher's test for frequencies of <5 and the Student's t-test for quantitative variables. The Mann–Whitney U test was used to determine the heterogeneity of 2 ordinal samples in the case of non-parametric variables of independent samples. The ANOVA test was used for variables with more than 2 categories. Analyses were performed using statistical software (SPSS version 19, IBM Corp., Armonk, NY).
ResultsNumber of Patients Registered vs Number Expected According to Deficiency PrevalenceThe population included in the REDAAT consisted of 511 individuals, of whom 469 (91.8%) were adults as the time of diagnosis and 42 (8.2%) were children.9 Of these, 348 had Pi*ZZ phenotype. This number represents approximately 3% of the expected cases according to epidemiological estimates for AATD in Spain.10
Participating Centers And DoctorsPatients were registered by 124 doctors in 103 healthcare facilities. Distribution of the centers were: 97 hospitals from the National Health System (94.2%), 3 primary care centers (2.9%), 2 private specialist clinics (1.9%) and 1 private hospital (1%). The 10 centers which registered the greatest number of cases were public hospitals authorized to teach medical students up to post-graduate level.
The mean number of cases registered by each doctor was 4 (SD: 8). However, 26.2% of the total patient population were registered by 3 doctors, who included 48, 44, and 42 cases each, from third-level university hospitals with lung transplantation programs. Distribution of cases registered by autonomous community is shown in Table 1.
Geographical Distribution of Registered Cases.
| AC | Cases Registereda | Population AC | Registration Rateb |
|---|---|---|---|
| Andalusia | 40 (7.8) | 8449985 | 0.5 |
| Aragon | 3 (0.6) | 1349467 | 0.2 |
| Asturias | 38 (7.4) | 1077360 | 3.5 |
| Balearic Islands | 3 (0.6) | 1119439 | 0.3 |
| Canary Islands | 28 (5.5) | 2118344 | 1.3 |
| Cantabria | 40 (7.8) | 593861 | 6.7 |
| Castile-La Mancha | 6 (1.2) | 2121888 | 0.3 |
| Castile-Leon | 52 (10.2) | 2546078 | 2.0 |
| Catalonia | 112 (21.9) | 7570908 | 1.5 |
| Extremadura | 4 (0.8) | 1108130 | 0.4 |
| Galicia | 63 (12.3) | 2781498 | 2.3 |
| Madrid | 68 (13.3) | 6498570 | 1.0 |
| Murcia | 2 (0.4) | 1474449 | 0.1 |
| Navarre | 9 (1.8) | 644566 | 1.4 |
| Basque Country | 23 (4.5) | 2193093 | 1.0 |
| Valencia | 20 (3.9) | 5129266 | 0.4 |
| Total | 511 (100) | 46776902 | 1.1 |
La Rioja and the autonomous cities of Ceuta and Melilla are not represented in this table as no cases were registered in any of these regions.
AC: autonomous community.
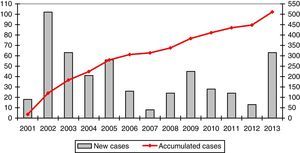
The mean number of cases registered per year was 39.3 (range: 8–102). The registration rate is represented in a graph in Fig. 1.
The percentage of correctly completed forms was high for most variables except for lung function parameters. Data reporting by variables is shown in Table 2.
Description of Data Reporting by Variables.
| Variable | Collected | % | |
|---|---|---|---|
| Demographic variables | Initial | 511 | 100 |
| Date of birth | 511 | 100 | |
| Sex | 511 | 100 | |
| Height | 489 | 96 | |
| Weight | 481 | 94 | |
| Clinical variables | Reason for determination | 510 | 100 |
| Phenotype | 511 | 100 | |
| Date of diagnosis | 464 | 91 | |
| Clinical presentation | 511 | 100 | |
| Age at onset of symptoms | 362 | 71 | |
| Main symptom | 439 | 86 | |
| Treatment | COPD medication | 511 | 100 |
| CHO | 511 | 100 | |
| Have they ever received RT? | 511 | 100 | |
| Did they stop treatment? | 511 | 100 | |
| Lung transplantation | 511 | 100 | |
| LFT | FEV1 preBD baseline (L) | 462 | 90 |
| FVC preBD baseline (L) | 441 | 86 | |
| FEV1 postBD baseline (L) | 427 | 84 | |
| FVC postBD baseline (L) | 408 | 80 | |
| FEV1 preBD follow-up (L) | 369 | 72 | |
| FVC preBD follow-up (L) | 366 | 72 | |
| FEV1 postBD follow-up (L) | 390 | 76 | |
| FVC postBD follow-up (L) | 386 | 76 | |
| KCO (%) | 144 | 28 | |
| Other variables | Liver enzyme tests | 511 | 100 |
| Working situation | 485 | 95 | |
| Death (yes/no) | 511 | 100 | |
| Total | 511 |
BD: bronchodilator; CHO: chronic home oxygen therapy; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; KCO: Krogh index or transfer coefficient (DLCO/VA); LFT: lung function tests; RT: replacement therapy.
Follow-up data were available for 225 individuals (44%). Median patient follow-up was 2 entries (interquartile range [IQR]: 1–4), and median follow-up time was 36 months [IQR: 12–84]. Investigators who registered a greater number of cases reported follow-up of their patients more frequently, and accumulated a longer follow-up period (mean 64.4 months for investigators with more than 40 cases compared to 34.7 months for investigators with 1–2 cases). Both differences were statistically significant (P=.001).
Characteristics of Patients Included in the Spanish Registry of Patients with Alpha-1 Antitrypsin DeficiencyA total of 448 adult patients were carriers of the Pi*ZZ or Pi*SZ phenotypes (348 Pi*ZZ and 100 Pi*SZ), and the rest were carriers of rare variants. Mean age at the time of diagnosis was 47.3 years (12.7) and mean age at the time of performing the analysis was 57.4 years (12.1). Aside from the symptoms that prompted the AATD study, a total of 364 (81%) of the adult patients presented some type of lung disease, particularly the Pi*ZZ population: 305 (87.6%) vs 59 (59%) of the Pi*SZ (P<.001). The 348 Pi*ZZ adults represented 74.2% of all cases registered. There were 269 (77.2%) index cases, while 62 (17.8%) were non-index cases. The characteristics of the Pi*ZZ individuals divided into index and non-index cases are shown in Table 3. The index cases were older at the time of AATD diagnosis, had poorer lung function, and higher mortality.
Characteristics of the Pi*ZZ Individuals Divided Into Index and Non-index Cases.
| Variable | Index | Non-index | P |
|---|---|---|---|
| Mean agea | 58.9 (10.4) | 54 (12.2) | .002 |
| Sex (men) | 167 (65.2) | 27 (43.5) | .002 |
| BMI (kg/cm2) | 25 (4.1) | 25.6 (4) | .3 |
| Smoking habit | |||
| Never smoker | 30 (11.7) | 10 (16.1) | |
| Active smoker | 18 (7) | 6 (9.7) | .46 |
| Former smoker | 208 (81.3) | 46 (74.2) | |
| Age at diagnosis (years)a | 48.14 (10.6) | 38.49 (11.7) | <.001 |
| Age at onset of symptoms (years)* | 37.9 (13) | 36.7 (12.8) | .002 |
| Reason for determination | |||
| Lung disease | 256 (95.2) | – | |
| Liver disease | 13 (4.8) | – | |
| Family screening | – | 62 (100) | – |
| Clinical presentation | |||
| Chronic bronchitis | 121 (47.3) | 15 (24.2) | .001 |
| Emphysema | 229 (89.5) | 30 (48.4) | <.001 |
| Asthma | 43 (16.8) | 14 (22.6) | .29 |
| Bronchiectasis | 88 (34.4) | 18 (29) | .42 |
| Other | 28 (10.9) | 5 (8.1) | .51 |
| Main symptom | |||
| Non-productive cough | 6 (2.5) | 4 (9.5) | |
| Productive cough | 37 (15.6) | 4 (9.5) | |
| Dyspnea at rest | 10 (4.2) | 0 | <.001 |
| Dyspnea on exertion | 171 (72.2) | 20 (47.6) | |
| Dyspnea attack | 11 (4.6) | 4 (9.5) | |
| Asymptomatic | 2 (0.8) | 10 (23.8) | |
| History of pneumonia | 83 (30.9) | 9 (14.5) | .005 |
| Bronchiectasis | 88 (32.7) | 18 (29) | .42 |
| FEV1 mean baseline (L) | 1.58 (0.7) | 2.87 (1.2) | <.001 |
| FEV1 mean baseline (%) | 47.4 (23.5) | 76.2 (32.6) | <.001 |
| Medication for lung disease | 232 (86.2) | 26 (41.9) | <.001 |
| Oxygen therapy | 44 (16.4) | 4 (6.5) | .03 |
| Replacement therapy | 139 (51.7) | 16 (25.8) | <.001 |
Data expressed as n (%).
BMI: body mass index; FEV1: forced expiratory volume in 1s.
In total, 158 (35.3%) patients received replacement therapy with intravenous AAT at some point. Characteristics of patients who received or did not receive replacement therapy are shown in Table 4. Patients who received treatment had poorer lung function and more symptoms at the time of registration.
Characteristics of Adult Pi*ZZ Population by Replacement Therapy Prescription.
| Variable | No RT | RT | P |
|---|---|---|---|
| Mean age (years)a | 56.8 (12.9) | 58.7 (8.2) | .15 |
| Sex (men) | 106 (55.8) | 107 (67.7) | .02 |
| BMI (kg/cm2) | 25.5 (3.7) | 24.6 (4.2) | .09 |
| Smoking habit | |||
| Never smoker | 34 (17.9) | 17 (10.8) | |
| Active smoker | 17 (8.9) | 8 (5.1) | .046 |
| Former smoker | 139 (73.2) | 133 (84.2) | |
| No. of cigarettes/daya | 18.9 (9.4) | 22.3 (10.3) | .013 |
| Age at diagnosis (years)a | 46.8 (13.2) | 46.2 (9.7) | .65 |
| Age at onset of symptoms (years)a | 38.6 (14.5) | 37.1 (12) | .36 |
| Reason for determination | |||
| Lung disease | 117 (61.6) | 139 (88) | |
| Liver disease | 11 (5.8) | 2 (1.3) | <.001 |
| Other | 4 (2.1) | 1 (0.6) | |
| Family screening | 46 (24.2) | 16 (10.1) | |
| Population screening | 2 (1.1) | 0 | |
| Other | 9 (4.7) | 0 | |
| Not available | 1 (0.5) | 0 | |
| Clinical presentation | |||
| Chronic bronchitis | 62 (32.6) | 81 (51.3) | <.001 |
| Emphysema | 124(65.3) | 149 (94.3) | <.001 |
| Asthma | 36 (18.9) | 24 (15.2) | .36 |
| Bronchiectasis | 60 (31.6) | 52 (32.9) | .79 |
| Main symptom | |||
| Non-productive cough | 5 (3.9) | 4 (2.8) | |
| Productive cough | 28 (18.1) | 14 (9.8) | <.001 |
| Dyspnea at rest | 6 (3.9) | 5 (3.5) | |
| Dyspnea on exertion | 90 (58.1) | 111 (77.6) | |
| Dyspnea attack | 8 (5.2) | 9 (6.3) | |
| Asymptomatic | 17 (11) | 0 | |
| History of pneumonia | 48 (25.3) | 47 (29.7) | .35 |
| Bronchiectasis | 60 (31.6) | 52 (32.9) | .79 |
| FEV1 mean baseline (L)a | 2.3 (1.1) | 1.5 (0.6) | <.001 |
| FEV1 mean baseline (%) | 73.5 (30.6) | 42.2 (20.2) | <.001 |
| Medication for lung disease | 128 (67.4) | 142 (89.9) | <.001 |
| Oxygen therapy | 19 (10) | 30 (19) | <.016 |
| Transplant | 3 (1.6) | 11 (7) | .011 |
| Death | 13 (6.8) | 41 (25.9) | <.001 |
Data expressed as n (%).
BMI: body mass index; FEV1: forced expiratory volume in 1s; RT: replacement therapy.
The REDAAT registry collects clinical and functional data from more than 500 individuals with AATD, principally Pi*ZZ and Pi*SZ phenotype carriers who mostly have lung disease. Thirty-five percent of them received AAT replacement therapy and follow-up data are available for about half of all cases. This underreporting of follow-up may be due to various reasons: asymptomatic patients with no manifestations of lung or liver disease do not generally attend regular clinic visits, and candidates for transplantation are referred to transplantation units and may lose contact with the pulmonologist who diagnosed them in their center of origin. Registry adherence by professionals varies for different reasons, but changes can be expected over the 20-year period since it was initiated, and many cases have been lost to follow-up as circumstances change for both doctors and patients (retirements, transfers, hospital mergers, changes in address, etc.).
Analysis of the REDAAT data shows a wide disparity in the proportion of cases registered by each doctor, and the geographical distribution of registered cases is also very irregular. These variations cannot be explained by differences in population, prevalence of the deficiency, or availability of health resources in the different autonomous communities. Indeed, the variations appear to correspond more to the investigator's profile and the size of the center than to the prevalence of AATD in each geographical area. The database contains good quality information and is a useful tool for the study of patient characteristics and the natural history of AATD.
A marked trend was observed toward accumulation of cases in third-level hospitals. This may be associated with the availability of diagnostic techniques, the severity of the lung disease, and the age of patients with pulmonary involvement, who may be candidates for inclusion in transplantation programs. Eighteen percent of registered individuals were seen in the center in which REDAAT was set up, which was also the first hospital in Spain to use replacement therapy.3,4 This suggests that a continuing interest in this disease in this center leads to higher rates of diagnosis, although this may also be the effect of larger numbers of patients being referred by other specialists to this center.
Centers which register most cases also seem to update the data more regularly. One possible reason for this is that, although a growing number of cases are now detected in smaller centers, this is a rare disease and the impact on functional status can be severe, so patients are likely to be referred to other higher level facilities, and the doctor who made the initial diagnosis does not have ready access to follow-up data.
No reliable epidemiological data are currently available from the National Health System specifying the real number of diagnosed individuals, so we cannot be sure that the sample included in the REDAAT is representative, nor can we rule out bias in the selection of the patients registered. So, while we can assume that not all diagnosed cases are registered, we can only estimate the rate of underdiagnosis in Spain using the data presented here, and these show that information is available on only a small percentage of the estimated AATD population based on prevalence studies.
There are an estimated 12000 Pi*ZZ carriers in Spain, of which 471 (3.9%) were registered in the REDAAT as of December 2015. This percentage is higher than that calculated by Stoller (2.4%) from the AIR data and the American registry.11,12 It has also been estimated that of the 12000 carriers of the Pi*ZZ genotype, 2500 (21%) may have COPD. In REDAAT, 440 Pi*ZZ carriers with COPD are registered, accounting for about 18% of estimated COPD-ZZ.10
While it is obviously of interest to determine the disease course of all AATD patients due to the variable clinical expression of this disease, it is particularly important to expand our knowledge of the patient subgroup that presents with lung disease, as this is the type that impacts most on morbidity and mortality. In this regard, our population is valuable, as it includes a high percentage of individuals with lung disease with an emphysema phenotype, particularly COPD, followed by the chronic bronchitis profile. With regard to the most frequent phenotypes, Pi*ZZ individuals, including those with lower accumulated tobacco consumption, had poorer lung function than Pi*SZ patients.9,13
In terms of data integrity, a high percentage of variables were correctly recorded, except for functional parameters in follow-up. This is indicative of a good database design and good real time data quality control since the website was updated in 2008.5 Since then, every new case forwarded to the registry is reviewed by the administrator before validation and definitive inclusion in the database. Errors or incomplete data can be very quickly detected and corrected.
The design of the REDAAT database has some limitations. The simplicity of the form means that data collection is quick (less than 10minutes), but there is no opportunity to include detailed information about some important aspects. Lack of information on the medication used, radiological findings to confirm the diagnosis of pneumonia, bronchiectasis or emphysema extension, confirmation of the quality or standardization of spirometries may be considered weaknesses that could reduce the reliability of the results. Therefore, the conclusions reached from an analysis of the database are not as solid as those that would be drawn from a prospective clinical trial or study specifically designed to evaluate these parameters. However, the data collected provide information on patient profiles and generate very important data for the potential design of more detailed studies on certain aspects of AATD.
A mean of 39 cases are registered annually, but the rate per year varies enormously. This is largely due to the different stages in the development of REDAAT. The online registry was launched in 2001, and the cases previously recorded on paper were transferred to the electronic database.8 Between 2007 and 2008 the server was changed and the website that hosted the database was updated, as a result of which the registry was not operational for several months. Between 2012 and 2013, SEPAR led an awareness-raising campaign as part of its SEPAR Year for Orphan Diseases that included activities specifically aimed at AATD, and this may have contributed to a greater number of entries.
In our population, onset of symptoms generally occurs between the 3rd and 4th decade of life, as also described by other authors.14 The delay between onset of symptoms and diagnosis of deficiency is almost 10 years on average. This finding is very similar to data provided by the American registry.15 However, the fact that COPD is greatly underdiagnosed and most AATD patients have this disease could have a cumulative effect: delay in COPD diagnosis added to the failure to suspect AATD means that advanced stages of emphysema are reached.16
Our study revealed that a high number of the cases registered as non-index had lung disease, but the possible existence of AATD was not suspected until it was detected in another family member. This finding reflects a continued lack of awareness or knowledge of AATD among health professionals, despite recommendations from institutions such as the World Health Organization and scientific societies.5,17–19
Replacement therapy was administered to 45.4% of the patients at some time since inclusion in the REDAAT registry. These patients more frequently presented emphysema and chronic bronchitis, they were more symptomatic and had worse lung function, higher mortality and indication for transplantation. In these patients, greater severity was associated with criteria for starting therapy, but no conclusions can be drawn as regards treatment efficacy. However, data on the efficacy of therapy were retrieved from the American registry, which was designed for this purpose, and provided information on 1048 individuals, with a follow-up of 3.5–7 years. A significant reduction in mortality was detected and FEV1 decreased more gradually in patients with FEV1 of 35%–49% who received continuous or intermittent treatment.20
ConclusionsREDAAT is a useful tool for studying the reality of AATD in Spain. The registry data are reasonably complete, although few participating doctors participate for long enough to allow follow-up data to be entered. The age of the patient cohort is similar to that of cohorts included in other registries, and most individuals have lung disease.
Conflict of InterestsBeatriz Lara has received fees for scientific consultancy and/or for speaking engagements from Bayer, Glaxo Smith Kline, Boehringer Ingelheim, Novartis, Grifols, Talecris, Menarini, Laboratorios Rovi and Pfizer.
Francisco Casas has received fees for scientific consultancy and/or for speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Pfizer, Teva, Menarini, Novartis, Gebro Pharma and Takeda.
Sergio Cadenas has received fees for speaking engagements from Almirall, Astra Zeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols and Novartis.
Sergio Curi has received fees for speaking engagements from Boehringer Ingelheim, Almirall and GlaxoSmithKline.
Francisco Dasi has received fees for speaking engagements from Grifols.
Marc Miravitlles has received fees for scientific consultancy and/or for speaking at conferences organized by Almirall, AstraZeneca, Boehringer Ingelheim, CSL Behring, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Teva, Cipla, Menarini, Novartis, Gebro Pharma and Takeda.
We thank the 124 doctors who over the years have dedicated their time to entering the data on their patients in the REDAAT registry; without them, this study would have been impossible.
We also thank Dr. Ferran Barbé for his contributions to this project and to the manuscript.
Please cite this article as: Lara B, Blanco I, Martínez MT, Rodríguez E, Bustamante A, Casas F, et al. Registro español de pacientes con déficit de alfa-1 antitripsina: evaluación de la base de datos y análisis de la población incluida. Arch Bronconeumol. 2017;53:13–18.