Although tobacco smoke is the main risk factor for chronic obstructive pulmonary disease (COPD), other inhaled toxics have also been associated with the disease. The present study analyzes data from exposure to these substances in a cohort of patients with COPD and assesses their impact on the clinical presentation of the disease.
MethodsThis is a cross-sectional analysis of the Clinical presentation, diagnosis and course of chronic obstructive pulmonary disease (On-Sint) study. All patients were smokers or ex-smokers as per protocol. In addition, during the inclusion visit patients were enquired about their occupational and biomass exposure history. The clinical features of patients with and without an added risk factor to tobacco were compared and those significant were entered in a multivariate logistic regression analysis, expressed as odds ratio (OR).
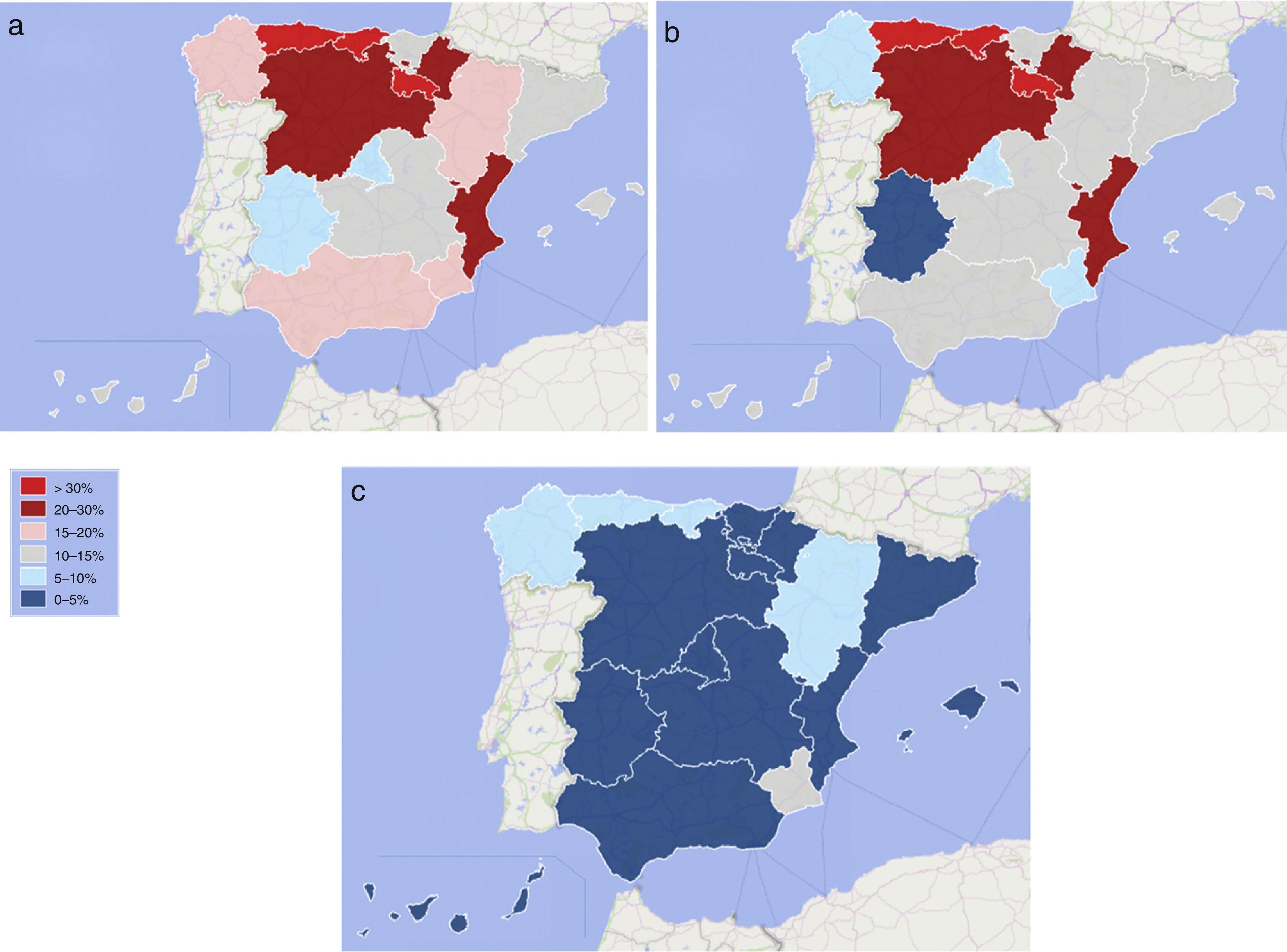
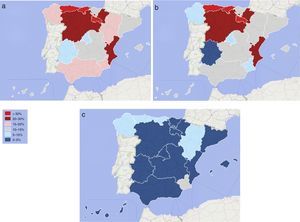
ResultsThe sample size was 1214 patients with COPD, of which 1012 (83.4%) had tobacco as the only risk factor and 202 (16.6%) had additional ones, mainly 174 (14.3%) with occupational gases and 32 (2.6%) with biomass exposure. The geographical distribution of this exposure showed a preference for the northern parts of the country and the East coast. The biomass exposure was rather low. Male gender (OR: 2.180), CAT score (OR: 1.036) and the use of long-term oxygen therapy (OR: 1.642) were associated with having an additional risk factor in the multivariate analysis.
ConclusionsOccupational exposures are more common than biomass in Spain. COPD caused by tobacco plus other inhalants has some differential features and a more impaired quality of life.
Aunque el humo del tabaco es el principal factor de riesgo de la enfermedad pulmonar obstructiva crónica (EPOC), también se han relacionado con la enfermedad otros agentes tóxicos inhalados. El presente estudio analiza datos de la exposición a estas sustancias y evalúa su impacto sobre la presentación clínica de la enfermedad en una cohorte de pacientes con EPOC.
MétodosSe trata de un análisis transversal del estudio Presentación clínica, diagnóstico y evolución de la enfermedad pulmonar obstructiva crónica (On-Sint). De conformidad con el protocolo, todos los pacientes eran fumadores o exfumadores. Durante la visita de inclusión se interrogó a los pacientes acerca de sus antecedentes de exposición laboral a tóxicos y a combustión de biomasa. Las características clínicas de los pacientes que presentaban algún factor de riesgo además del tabaco se compararon con las de los pacientes que no presentaban factores de riesgo adicionales, y los factores que indicaron ser significativos fueron incluidos en un análisis de regresión logística multivariante, expresados en oportunidades relativas (odds ratio [OR]).
ResultadosLa muestra incluyó 1.214 pacientes con EPOC, en 1.012 (83,4%) de los cuales el tabaco era el único factor de riesgo. En 202 (16,6%) se constataron otros factores, en 174 (14,3%) principalmente la exposición a gases en el ámbito laboral y en 32 (2,6%) la exposición a combustión de biomasa. La distribución geográfica de esta exposición fue mayor en la zona norte y la costa este del país. La exposición a humo de biomasa fue relativamente baja. El análisis multivariante mostró asociaciones entre la presentación de un factor de riesgo adicional y el sexo masculino (OR: 2,180), la puntuación CAT (OR: 1,036) y el uso de oxigenoterapia crónica (OR: 1,642).
ConclusionesEn España, la exposición laboral a tóxicos inhalados es más frecuente que la exposición a humo de biomasa. La EPOC causada por el tabaco y otros productos inhalados tiene algunas características diferenciales y provoca un mayor deterioro de la calidad de vida.
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with exposure to noxious particles or gases.1 Two key findings are needed for a diagnosis of COPD: non-reversible airflow obstruction and exposure to inhaled particles or gases causing this obstruction. The main inhaled gas associated with COPD is tobacco smoke.2 However, other inhaled toxins have also been described as a risk factor for COPD.3
Two main substances other than tobacco have been implicated in COPD, namely biomass and occupational dust or chemicals. The term biomass defines the energy obtained from the combustion of organic products. This combustion produces fumes that can be inhaled, causing bronchial inflammation in susceptible individuals, and secondary bronchial obstruction, indistinguishable from COPD in many respects.4 Furthermore, occupational exposure to dust, gases or volatile chemicals can also cause COPD.5 However, it is common to find patients who besides being exposed to these substances also smoke or have smoked, so it is sometimes difficult to determine to what extent each substance has contributed to the development of COPD; moreover, both substances have an additive risk factor for COPD.6
It is frequently reported in the literature that biomass is the most frequently inhaled substance after tobacco smoke in developing countries.7 However, the frequency of exposure depends on the geographical area where it is studied, since the use of biomass differs depending on the region studied. In Spain, biomass and occupational dust exposures have not been fully described and only 1 study has provided some information about exposure to substances other than tobacco in a population of patients with COPD.8 This study analyzes the information from the On-Sint study, presents data on exposure to substances other than tobacco in this cohort of COPD patients and assesses their impact on the clinical presentation of the disease.
MethodsThis study is a cross-sectional analysis of the On-Sint study. The methodology of the On-Sint study has been extensively described elsewhere.9 Briefly, this is an observational, nationwide, real-life, cohort study, in which patients diagnosed with COPD were recruited between December 2011 and April 2013 by Primary Care (PC) and Secondary Care (SC) physicians. Consecutive patients aged >40 years who were smokers or ex-smokers with a history of >10 pack-years, diagnosed with COPD, with a complete clinical history of respiratory symptoms, able to complete the CAT questionnaire, and who gave their written informed consent were selected to participate in the study. Ethical approval was granted by the Institutional Review Board from Servicio Gallego de Salud (SERGAS) registry number 2011/359. In order to record real-life clinical behavior of participant doctors, the only exclusion criterion considered in the study protocol was participation in any other clinical trial at the time of inclusion. In addition, patients with pulmonary diseases associated with occupational factors including occupational asthma, hypersensitivity pneumonia or interstitial lung diseases were also excluded. In order to make a real-life evaluation, patients were recruited by PC and SC physicians with no matching for gender, age, lung function or any clinical features. Sample size was calculated according to the prevalence and the degree of underdiagnosis of COPD in Spain.10 A planned total of 1440 patients with COPD was expected to constitute a sample of 0.1% of the study population, assuming 10% of patients would have no valid information. Although a uniform distribution of recruitment within the country was planned including all regions in the country, the selection of participant investigators was voluntary, with no attempt to achieve representative sampling.
During the inclusion visit, patients underwent a clinical evaluation, which included the presence of risk factors for COPD, not only tobacco smoke, but also other inhaled substances. Smoking history was collected, including current smoking status and cumulative consumption in pack-years. Occupational and biomass exposure was self-reported. Patients were asked about their previous exposures. In particular, they were asked if they had been exposed to any other risk factor and, if they replied in the affirmative, the exact exposure was determined. Exposure to other substances was categorized into three groups: occupational dust and chemicals, biomass fuels, or other exposures. The participating centers were classified according to the population of the locality where they were in rural (<5,000 inhabitants), semiurban (5,000–19,999 inhabitants) and urban (≥20,000 inhabitants).
The CAT questionnaire was administered to all participants in the inclusion visit. The questionnaire was self-administered or administered by the investigator if any patient had reading, writing or sight difficulties. If more than two questions were unanswered, the questionnaire was considered invalid.
Statistical ComputationsAlthough cases were recruited by respiratory and primary care physicians, all patients were followed up by general practitioners, so comparison between PC and SC was impractical and would have produced confounding results. Consequently, all patients were analyzed together. In addition, since the sample was not intended to be representative of the different regions, we did not compare the impact of different exposures by regions. Statistical computations were performed using the Statistical Package for Social Sciences (SPSS, IBM Corporation Somers, NY, US), version 20.0. Absolute and relative frequencies for categorical questions were used to describe variables, together with the inter-regional range (IRR), expressing the range of the means or percentages within the different regions, allowing us to further assess heterogeneity. The IRR is expressed as a percentage for qualitative variables, while quantitative variables are expressed by unit of measure. Geographical maps were constructed using Microsoft PowerMap for Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA). Bivariate analyses comparing cases with tobacco smoke as the only risk factor with those with additional risk factors were performed with the chi-squared test or the unpaired Student T test, after checking the equality of the variances with the Levene test. Variables with a P value <.1 were entered into a backward stepwise multivariate binomial logistic regression analysis with the presence of additional risk factors as the dependent variable. A P value <.05 was considered significant.
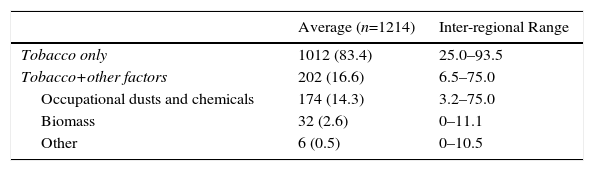
ResultsDuring the study period, 1264 patients were included in the study. Of these, 50 (4.0%) patients were excluded for not fulfilling the inclusion criteria. Thus, the sample size of the On-Sint cohort was 1214 patients with COPD, of which 857 (70.6%) were recruited by PC and 357 (29.4%) by SC physicians. The frequency of the different risk factors for COPD is depicted in Table 1. All patients were smokers or ex-smokers as per the inclusion criteria. However, 1012 (83.4%) had tobacco as the only risk factor whereas 202 (16.6%) had an additional exposure. The geographical distribution of this exposure is depicted in Fig. 1, showing greater exposure in the northern parts of the country and on the east coast. Biomass exposure was rather low.
Prevalence of the Different COPD Risk Factors.
| Average (n=1214) | Inter-regional Range | |
|---|---|---|
| Tobacco only | 1012 (83.4) | 25.0–93.5 |
| Tobacco+other factors | 202 (16.6) | 6.5–75.0 |
| Occupational dusts and chemicals | 174 (14.3) | 3.2–75.0 |
| Biomass | 32 (2.6) | 0–11.1 |
| Other | 6 (0.5) | 0–10.5 |
Average data expressed as mean (standard deviation) or absolute (relative) frequencies depending on the nature of the variable. The inter-regional range is expressed as percentage for qualitative variables, while quantitative variables are expressed in its unit of measure.
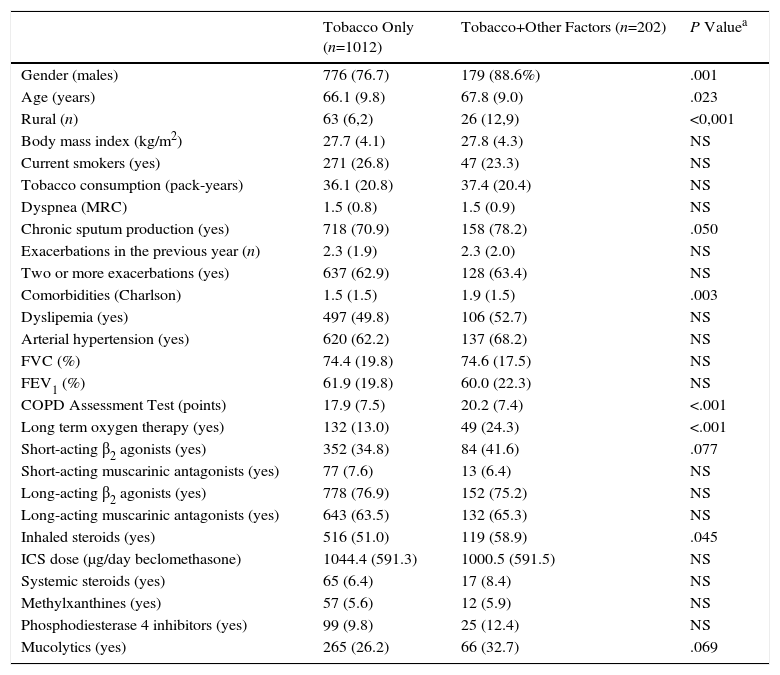
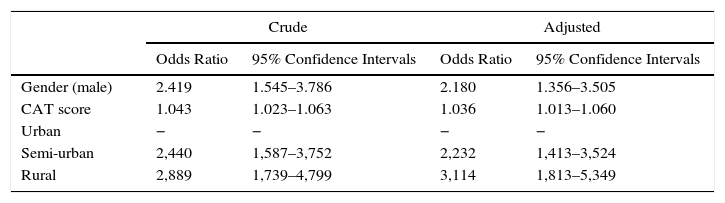
The differences between COPD with tobacco as the only risk factor versus COPD with additional risk factors are summarized in Table 2. The main factors associated with having an added risk factor were gender, age, chronic sputum production, the Charlson index, CAT score, long-term oxygen therapy, short-acting β2 agonists, inhaled steroids, and the use of mucolytics. The results of the multivariate analysis including these variables are presented in Table 3. Male gender, CAT score and rurality were associated with having an additional risk factor.
Differences Between Cases With Tobacco Smoke as the Only Risk Factor Versus Cases With Additional Factors.
| Tobacco Only (n=1012) | Tobacco+Other Factors (n=202) | P Valuea | |
|---|---|---|---|
| Gender (males) | 776 (76.7) | 179 (88.6%) | .001 |
| Age (years) | 66.1 (9.8) | 67.8 (9.0) | .023 |
| Rural (n) | 63 (6,2) | 26 (12,9) | <0,001 |
| Body mass index (kg/m2) | 27.7 (4.1) | 27.8 (4.3) | NS |
| Current smokers (yes) | 271 (26.8) | 47 (23.3) | NS |
| Tobacco consumption (pack-years) | 36.1 (20.8) | 37.4 (20.4) | NS |
| Dyspnea (MRC) | 1.5 (0.8) | 1.5 (0.9) | NS |
| Chronic sputum production (yes) | 718 (70.9) | 158 (78.2) | .050 |
| Exacerbations in the previous year (n) | 2.3 (1.9) | 2.3 (2.0) | NS |
| Two or more exacerbations (yes) | 637 (62.9) | 128 (63.4) | NS |
| Comorbidities (Charlson) | 1.5 (1.5) | 1.9 (1.5) | .003 |
| Dyslipemia (yes) | 497 (49.8) | 106 (52.7) | NS |
| Arterial hypertension (yes) | 620 (62.2) | 137 (68.2) | NS |
| FVC (%) | 74.4 (19.8) | 74.6 (17.5) | NS |
| FEV1 (%) | 61.9 (19.8) | 60.0 (22.3) | NS |
| COPD Assessment Test (points) | 17.9 (7.5) | 20.2 (7.4) | <.001 |
| Long term oxygen therapy (yes) | 132 (13.0) | 49 (24.3) | <.001 |
| Short-acting β2 agonists (yes) | 352 (34.8) | 84 (41.6) | .077 |
| Short-acting muscarinic antagonists (yes) | 77 (7.6) | 13 (6.4) | NS |
| Long-acting β2 agonists (yes) | 778 (76.9) | 152 (75.2) | NS |
| Long-acting muscarinic antagonists (yes) | 643 (63.5) | 132 (65.3) | NS |
| Inhaled steroids (yes) | 516 (51.0) | 119 (58.9) | .045 |
| ICS dose (μg/day beclomethasone) | 1044.4 (591.3) | 1000.5 (591.5) | NS |
| Systemic steroids (yes) | 65 (6.4) | 17 (8.4) | NS |
| Methylxanthines (yes) | 57 (5.6) | 12 (5.9) | NS |
| Phosphodiesterase 4 inhibitors (yes) | 99 (9.8) | 25 (12.4) | NS |
| Mucolytics (yes) | 265 (26.2) | 66 (32.7) | .069 |
Data expressed as mean (standard deviation) or absolute (relative) frequencies depending on the nature of the variable. MRC: Medical Research Council scale. ICS: inhaled steroids.
Multivariate Analysis of Factors Associated With Having an Additional Risk Factor.
| Crude | Adjusted | |||
|---|---|---|---|---|
| Odds Ratio | 95% Confidence Intervals | Odds Ratio | 95% Confidence Intervals | |
| Gender (male) | 2.419 | 1.545–3.786 | 2.180 | 1.356–3.505 |
| CAT score | 1.043 | 1.023–1.063 | 1.036 | 1.013–1.060 |
| Urban | − | − | − | − |
| Semi-urban | 2,440 | 1,587–3,752 | 2,232 | 1,413–3,524 |
| Rural | 2,889 | 1,739–4,799 | 3,114 | 1,813–5,349 |
CAT, COPD Assessment Text; LTOT, long-term oxygen therapy.
This is the first study to show the frequency of exposure to other risk factors in addition to tobacco, and the distribution within the different Spanish regions, showing a greater exposure in the northern part of the country and on the east coast. In addition, the study shows the clinical variables associated with this exposure, indicating that COPD caused by tobacco plus an additional risk factor has some similarities with traditional COPD, except that it is more frequent in men, has a greater impact on HRQL for a similar functional impairment, and is associated with rural areas.
Various studies have provided evidence that a considerable proportion of individuals with COPD present non-tobacco risk factors. The Burden of Obstructive Lung Disease (BOLD) Study evaluated data from 14 countries and found that among 4291 never smokers, 6.6% met criteria for mild COPD, and 5.6% met criteria for moderate to very severe disease.11 Occupational exposures leading to COPD have been described for several jobs. High exposure to gases or fumes was associated with chronic bronchitis12 and the relationship with lung function decline or chronic respiratory symptoms has been described in welders,6 agricultural workers,13,14 and different industries.15–17 A population-based study in north-east England investigated respiratory symptoms and the prevalence of spirometrically defined COPD between 2002 and 2004. The prevalence of respiratory symptoms was 55% and COPD was present in 10% of subjects. Interestingly, COPD and respiratory symptoms were associated with occupational exposures.18 In the USA the prevalence of COPD according to occupation has recently been reported for different occupational groups on the basis of the National Health Interview Survey Occupational Codes, with a prevalence of below 5% for all occupational exposures.19
The impact of biomass exposure is also well documented as a risk factor for COPD.4 Moreover, an improvement in lung function and prognosis has been shown when air pollution from biomass is reduced.20,21 However, biomass is used much less in a developed country like Spain. In our cohort, the frequency of occupational exposure was higher than reported for biomass exposure. In particular, the northern part of the country is more frequently exposed than the south, probably reflecting the differences in the distribution of industries throughout the country.
Our study has found some associations between additional smoke exposure and clinical characteristics. The association with male gender seems obvious since in Spain the jobs involving occupational exposure are mostly performed by men.22 A previous cross-sectional analysis reported that COPD with exposure to vapor, dust, gas or fumes at any time was associated with older age, male gender, work-related respiratory disability, current wheezing and hay fever, despite similar severity of airflow obstruction.23 However, while this is true for our community, in different regions in different countries including rural areas or agricultural settings, a relationship with female gender has been described.18,24 In addition, we found an association with a more impaired health status as measured by the CAT score, suggesting a higher impact of the disease for a similar lung function. Previous studies have also identified a relationship between occupational exposure and health status,25 so our data confirm previous associations in a cohort of patients from a country with specific characteristics with regard to non-tobacco COPD risk factor exposure.
Although we were not able to find any other associations, the type of COPD may also be influenced by the type of smoke. Previous studies have found that female patients with wood smoke-related COPD do not appear to develop emphysema as much as individuals with tobacco-related COPD, despite presenting severe airway involvement.26 In Mexico, it has also been reported that biomass smoke exposure is associated with less emphysema but more air trapping than tobacco smoke exposure, suggesting an airway-predominant phenotype.27 Unfortunately, the methodology used in the present study did not allow us to investigate these differences in the different respiratory compartments.
The CAT score is a self-administered health status questionnaire for assessing the impact of COPD on patients. Different studies have demonstrated its good psychometric properties and its association with several clinically relevant outcomes.28 Accordingly, CAT is able to capture part of the variability in the clinical impact of the disease which is not evaluated by lung function. In this study, we found that patients with additional exposures expressed a different impact of the disease as measured by CAT, suggesting that COPD with other risk factors may have a more profound impact for the same lung function impairment.
Several issues must be considered for the correct interpretation of our data. Occupational and biomass exposure was self-reported. Various validated questionnaires are available in the literature for evaluating the different exposures in a formal occupational interview.29 However, for the sake of simplicity we opted for self-reported recording of the information. Similarly, we recorded the history of exposure, but not the intensity of this exposure. A recent study has validated a biomass exposure index to quantify the amount of biomass exposure during a life-time.24 Additionally, we did not record the nature of the biomass or the different jobs, which may have provided extra information. Another aspect that merits consideration is that, in order to make the project affordable, we collected a limited number of variables for analysis. Thus, it is possible that future trials including a higher number of variables may yield a more comprehensive overview. Finally, the sample size was not estimated to allow representative geographical distribution sampling or to allow comparisons between regions. Consequently, the analysis did not attempt to make direct comparisons between the geographical areas within the country. Nonetheless, even with these limitations, we believe that the information presented here represents the best updated information available on different exposures in Spain.
ConclusionIn summary, our study shows the different distribution of exposure to other risk factors in addition to tobacco in the different Spanish regions, showing a greater exposure in the northern part of the country and the east coast. Our study shows the clinical variables associated with this exposure that characterize COPD caused by tobacco plus an additional risk factor. The information provided here could serve as a guide to alert health planners and doctors to the potential effects of these substances and take actions to improve prevention and early diagnosis.
FundingThis project was funded by an unrestricted grant from Novartis Farmacéutica, SA, Spain.
Conflict of InterestThe authors declare no conflicts of interest.
Please cite this article as: López-Campos JL, Fernández-Villar A, Calero-Acuña C, Represas-Represas C, López-Ramírez C, Leiro Fernández V, et al. Exposición laboral y a biomasa en la EPOC: resultados de un análisis transversal del estudio On-Sint. Arch Bronconeumol. 2017;53:7–12.