Influenza is a very common contagious disease that carries significant morbidity and mortality. Treatment with antiviral drugs is available, which if administered early, can reduce the risk of severe complications. However, many virus types develop resistance to those drugs, leading to a notable loss of efficacy. There has been great interest in the development of new drugs to combat this disease. A wide range of drugs has shown anti-influenza activity, but they are not yet available for use in the clinic. Many of these target viral components, which others are aimed at elements in the host cell which participate in the viral cycle. Modulating host components is a strategy which minimizes the development of resistance, since host components are not subject to the genetic variability of the virus. The main disadvantage is the risk of treatment-related side effects. The aim of this review is to describe the main pharmacological agents currently available and new drugs in the pipeline with potential benefit in the treatment of influenza.
La gripe es una enfermedad contagiosa altamente prevalente y con significativa morbimortalidad. El tratamiento disponible con fármacos antivirales, de ser administrado de forma precoz, puede reducir el riesgo de complicaciones severas; sin embargo, muchos tipos de virus desarrollan resistencia a estos fármacos, reduciendo notablemente su efectividad. Ha habido un gran interés en el desarrollo de nuevas opciones terapéuticas para combatir la enfermedad. Una gran variedad de fármacos han demostrado tener actividad antiinfluenza, pero aún no están disponibles para su uso en la clínica. Muchos de ellos tienen como objetivo componentes del virus, mientras que otros son dirigidos a elementos de la célula huésped que participan en el ciclo viral. Modular los componentes del huésped es una estrategia que minimiza el desarrollo de cepas resistentes, dado que estos no están sujetos a la variabilidad genética que tiene el virus. Por otro lado, la principal desventaja es que existe un mayor riesgo de efectos secundarios asociados al tratamiento. El objetivo de la presente revisión es describir los principales agentes farmacológicos disponibles en la actualidad, así como los nuevos fármacos en estudio con potencial beneficio en el tratamiento de la gripe.
Influenza is an infectious disease caused by various types of influenza virus, characterized by a highly contagious, acute respiratory syndrome. It usually presents in a mild form which resolves after 3–7 days, but it can also lead to other secondary infections or present in more severe forms, such as pneumonia or acute respiratory distress syndrome, which can be fatal, particularly in elderly patients.1–4 Seasonal influenza affects 5%–10% of the world's population every year, producing around 3–5 million severe cases and between 250000 and 500000 deaths. Pandemic outbreaks with high mortality rates can occur, impacting severely on public health.5
Vaccination is essential in preventing both the disease and complications, which primarily occur in risk groups such as children, elderly patients, patients with chronic respiratory disease and pregnant women. If treatment with antivirals is administered without delay, the risk of severe complications can be reduced; however, many virus strains develop pharmacological resistance and lose efficacy, so there has been great interest in recent years in developing new therapeutic options for combating the disease. This review article describes the main pharmacological agents currently available, and analyzes new medications under study that show potential benefit in the treatment of influenza5–7 (Table 1).
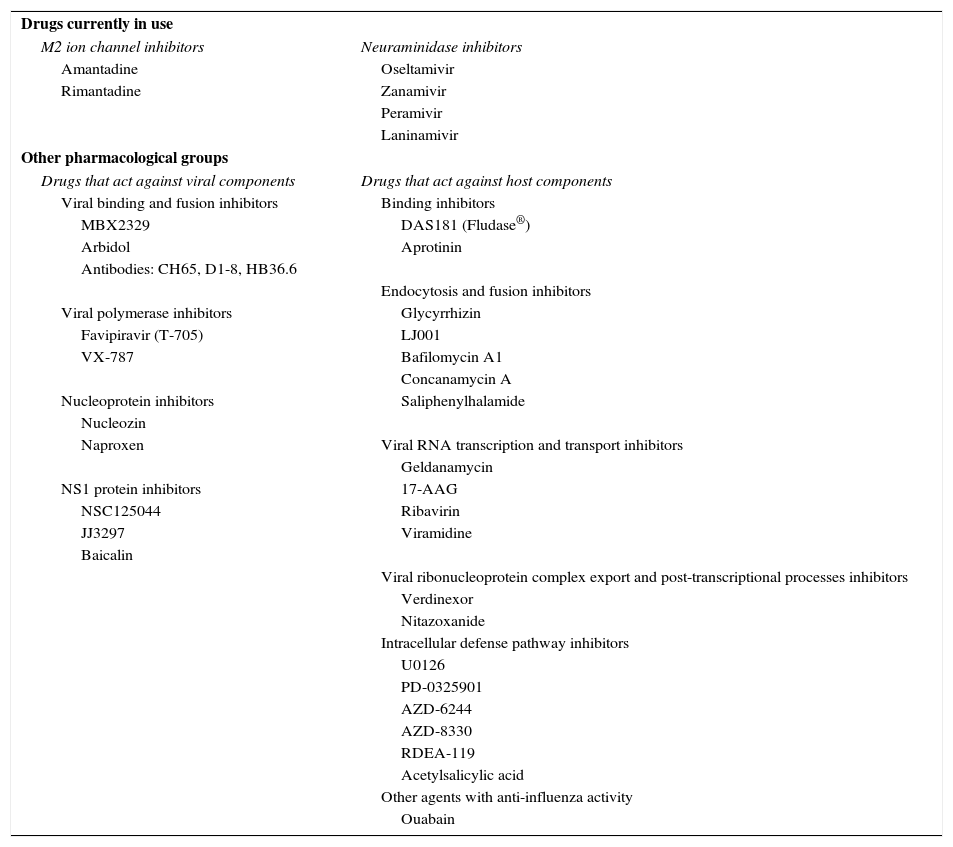
Anti-Influenza Drugs.
| Drugs currently in use | |
| M2 ion channel inhibitors | Neuraminidase inhibitors |
| Amantadine | Oseltamivir |
| Rimantadine | Zanamivir |
| Peramivir | |
| Laninamivir | |
| Other pharmacological groups | |
| Drugs that act against viral components | Drugs that act against host components |
| Viral binding and fusion inhibitors | Binding inhibitors |
| MBX2329 | DAS181 (Fludase®) |
| Arbidol | Aprotinin |
| Antibodies: CH65, D1-8, HB36.6 | |
| Endocytosis and fusion inhibitors | |
| Viral polymerase inhibitors | Glycyrrhizin |
| Favipiravir (T-705) | LJ001 |
| VX-787 | Bafilomycin A1 |
| Concanamycin A | |
| Nucleoprotein inhibitors | Saliphenylhalamide |
| Nucleozin | |
| Naproxen | Viral RNA transcription and transport inhibitors |
| Geldanamycin | |
| NS1 protein inhibitors | 17-AAG |
| NSC125044 | Ribavirin |
| JJ3297 | Viramidine |
| Baicalin | |
| Viral ribonucleoprotein complex export and post-transcriptional processes inhibitors | |
| Verdinexor | |
| Nitazoxanide | |
| Intracellular defense pathway inhibitors | |
| U0126 | |
| PD-0325901 | |
| AZD-6244 | |
| AZD-8330 | |
| RDEA-119 | |
| Acetylsalicylic acid | |
| Other agents with anti-influenza activity | |
| Ouabain | |
Influenza viruses belong to the Orthomyxoviridae family, and are classified as A, B or C. Influenza A viruses circulate in several species, including humans, horses and related animals, swine, and birds, while type B affects only humans. Influenza caused by types A and B is indistinguishable; in contrast, type C causes mild respiratory symptoms.8–10
The structure of influenza A virus consists of a lipid envelope that is generated from the host cell, to which hemagglutinin (HA) and neuraminidase (NA) glycoproteins are anchored. These surface antigens are used to classify the viruses (e.g., H1N1, N3N2, H5N1). The outer membrane also contains matrix proteins M2 and M1, while the center of the viral particle contains the ribonucleoprotein complex (segments of viral RNA and polymerase basic protein 1, polymerase basic protein 2 [PB 2], and polymerase acidic protein [PA]), nucleoprotein (NP), nuclear export protein, and non-structural protein 2. The genome consists of a single-stranded RNA chain with 8 segments, which produce between 8 and 12 viral proteins.11–16
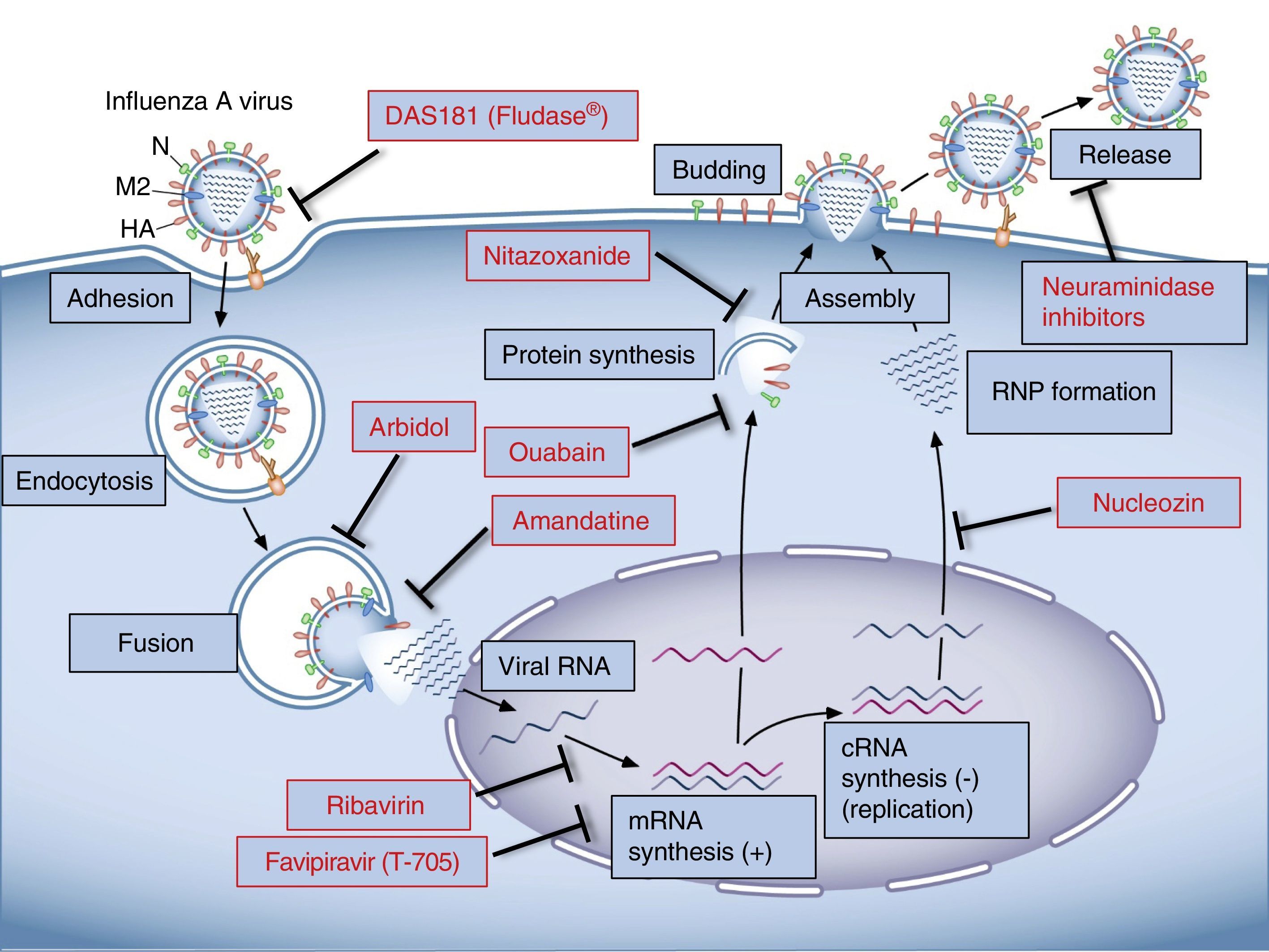
Viral CycleThe influenza virus anchors on the host cell when HA binds with the sialic acid of the glycoproteins or glycolipids on the cell membrane.17 The influenza species affecting humans selectively recognize the sialic acid linked to galactose by a α2,6 (SA α2,6Gal) linkage abundant in the epithelial cells of the respiratory tract.18,19 Once the virus binds to the membrane receptor, it enters the host cell by a process of endocytosis. It is released into the cell cytoplasma, and its membrane is fused to that of the endosome. Viral HA plays a key role in this process, because when it is cleaved by the host proteases20,21 a region known as “fusion peptide” is exposed. This interacts with the endosome membrane, resulting in the fusion of the membranes and the release of the contents of the virion into the cell cytoplasm.22–24 An important step in this process is endosome acidification which is mediated by the M2 ion channel protein. Protons enter through this channel and cause the M1 matrix protein to dissociate from the ribonucleoprotein complex of the virus, which is then released to the cytoplasm for subsequent nuclear importation.25–27 Once inside the nucleus, viral RNA is transcribed to messenger RNA (mRNA), which then undergoes the polyadenylation essential for the expression of viral proteins.28 Viral proteins are translated by the host cell machinery, and when the PA, PB and NP proteins have been synthesized, they are imported to the nucleus to facilitate the transcription and replication of the viral RNA. Ribonucleoprotein complexes are then exported from the nucleus, a process requiring non-structural protein 2, nuclear export protein and M1. The viral proteins HA, NA and M2 are glycosylated in the endoplasmic reticule and transported via the Golgi network to the cell membrane.39 The ribonucleoproteins and the 8 viral segments are transported to the cell membrane, where they are packaged to generate viral particle buds. Several structural viral proteins, such as HA, NA and M2, interact with the cell lipid membrane, thus contributing to the budding process.29 This process is completed when the membranes fuse in the base of the virus bud and the virus is shed by the action of NA, which catalyzes the elimination of sialic acid from the surface glycoproteins.30
TreatmentDrugs Currently in UseOf the 5 anti-influenza drugs currently available, only 3 are recommended by the U.S. Food and Drug Administration for this season: oral oseltamivir (Tamiflu®), inhaled zanamivir (Relenza®), and intravenous peramivir (Rapivab®), all of which are NA inhibitors.31 M2 ion channel inhibitors, amantadine and rimantadine, are not recommended as circulating viruses have a high rate of resistance of these compounds and are ineffective against type B and C influenza viruses.32
M2 Ion Channel InhibitorsAdamantane derivatives, such as amantadine and rimantadine, have known anti-influenza A activity, and were the first choice in the treatment of influenza for many years. They act by binding to a specific pocket on the viral M2 protein, stabilizing its closed conformation and preventing the virus from releasing the ribonucleoprotein complex to the cytoplasm after fusion, thus halting the viral cycle.33 Amantadine has also been shown to affect the pH of the vesicles that transport the viral glycoproteins, thus interfering with the assembly process.34
Viral resistance to these drugs has led to the development of new structurally related compounds, such as azoloadamantanes.35,36 However, none of the drugs in this group has demonstrated activity against all circulating amantadine-resistant viruses.
Neuraminidase InhibitorsThese compounds are active against influenza A and B; oseltamivir and zanamivir are the oldest, and peramivir and laninamivir belong to the new generation. These drugs prevent the cleavage of sialic acid, inhibiting virion release and preventing the dissemination of new viral particles to other cells.37 Less than 2% of worldwide circulating viruses in 2013–2014 were resistant to NA inhibitors; however, viruses that are highly resistant to these drugs have been identified in localized communities (Japan, China, Australia).38 New, promising NA inhibitor molecules are being developed with activity against strains which have shown resistance to currently available drugs.39–42
In clinical practice, NA inhibitors are the only anti-influenza drugs currently recommended; the most widely used of these is oseltamivir, given its good oral bioavailability. However, the interpretation of clinical studies that support its efficacy is highly controversial. A metaanalysis by the Cochrane group in 2014 revealed a modest benefit from oseltamivir treatment in cases of mild influenza, with reduced symptom duration from 7 to 6.3 days in adults and reduced symptoms in healthy children; however, no benefit was demonstrated in children with asthma. Hospitalization rates in patients receiving oseltamivir was similar to that in untreated patients.43 A metaanalysis published in the Lancet in 2015 found that for mild cases of confirmed influenza infection, oseltamivir treatment reduced duration of symptoms, lower respiratory tract infections, and hospital admissions. Both studies reported nausea and vomiting as the main side effects.44 While no randomized clinical trials have been performed in severe forms of influenza, observational studies suggest that oseltamivir significantly reduces mortality in serious cases.45–47 Clinical practice guidelines recommend the early initiation of antivirals for the treatment of severe cases of confirmed influenza that require hospitalization or in patients with a risk of serious complications, such as those aged less than 2 or more than 65 years, with chronic lung disease, immunosuppression or morbid obesity, and pregnant women or women who have given birth within less than 2 weeks. Post-exposure chemoprophylaxis can be considered in individuals with a high risk of complications or who have not been immunized, and should always be started within 48h of exposure. The first line of treatment is oseltamivir, while inhaled, nebulized or intravenous zanamivir is indicated for oseltamivir-resistant strains or in subjects with poor intestinal absorption. The indicated dose of oseltamivir is 75mg twice a day for treatment and once a day for chemoprophylaxis, while inhaled zanamivir should be administered at 10mg twice a day for treatment and once a day for chemoprophylaxis; zanamivir is not recommended in patients with asthma or COPD. Treatment must be administered for 5 days, but duration can be extended in severe cases; recommended prophylaxis duration is 10 days, for both oseltamivir and zanamivir.32,48,49
Other Pharmacological GroupsA considerable number of potential antiviral agents are currently being tested, but these are not yet available for use in the clinic. The most important groups are described below.
Drugs that Act Against Viral ComponentsInhibitors of Viral Binding and FusionSynthetic viral HA inhibitors: This group consists of a variety of molecules recognizable by the viral HA, thus interfering in virus–cell interaction. Examples of these are MBX2329 (an aminoalkyl phenyl ester) and MBX2546 (a sulfonamide), which inhibit influenza A virus entry by binding to the HA stem region, interfering with viral fusion.50 Other studies have used synthetic sialic acid analog peptides that are recognized by the viral HA and inhibit its action,51 and fusion peptide antagonists that interrupt the conformational change in HA needed for the correct fusion of the virus to the cell.52
Arbidol: A synthetic drug currently approved for the treatment of influenza in Russia and China, but not in the US, given the lack of clinical evidence.49 This is a hydrophobic molecule that penetrates the virus lipid membrane and interacts with the membrane phospholipid and transmembrane proteins enriched in aromatic residues from the viral envelope, interfering with entry and host cell fusion processes.53,54 A multicenter randomized clinical study (ARBITR) in influenza patients treated with arbidol showed a shorter disease duration with reduced severity and virus shedding.55
Resistance to arbidol has been identified in some influenza strains with HA2 subunit mutations that allow the virus to continue the process of fusing to the endosome membrane.56 The synthesis of structurally related compounds paves the way for the development of new antivirals, such as synthetic indoles that have shown greater antiviral potency than arbidol against certain influenza A subtypes in in vitro studies.57
Anti-HA antibodies: Monoclonal antibodies targeting highly conserved HA sites have been developed, permitting neutralization of influenza virus strains. For example, CH65 antibody obtained from a patient who received anti-influenza vaccination in 2007, has been shown to be effective in vitro against a wide spectrum of H1N1 influenza strains, due to its binding to the HA1 subunit receptor pocket.58 D1-8 antibody has shown efficacy in neutralizing different strains of H3N2 influenza virus in vitro and in vivo, achieving greater survival in mice compared to treatment with oseltamivir.59 Antibodies targeting the HA protein stem may also be effective against the influenza virus. HB36.6 showed efficacy against different strains of H1N1 and N5N1 in vitro, while in mice, it was able to reduce viral replication and improve survival.60
Viral Polymerase InhibitorsViral polymerase is a protein that remains highly conserved among the various influenza strains, making it a therapeutic target of interest.
Favipiravir (T-705) is a molecule that can convert to a nucleoside analog, ribonucleotide T-705-4-ribofuranosyl-5’-monophosphate, within the cell, inhibiting viral RNA polymerase activity without affecting cell synthesis of RNA or DNA.61 It has demonstrated efficacy against various influenza strains.62–64
PB2 inhibitors: A 5′ cap initiator is needed for the translation of mRNA, which the virus “steals” from host pre-mRNA. This process requires the PB2 subunit to bind to the 5′ cap of the host pre-mRNA, with subsequent cleavage of the 5′ end by the PA protein with endonuclease activity.65,66 Drugs that interfere with this process are currently under study and show great antiviral potential.67 One example is VX-787, which has shown in vitro activity against several classes of influenza A, and is effective both as prophylaxis and treatment.68,69
Nucleoprotein InhibitorsNucleoprotein binds to viral RNA and forms part of the ribonucleoprotein complex. It is essential for the synthesis of viral RNA and also participates in the nuclear export of viral ribonucleoproteins and in cytoplasmic trafficking.70
Nucleozin: This is the most widely studied drug of the group. It has demonstrated antiviral activity at various stages of the viral cycle: at early stages it inhibits viral RNA transcription and protein synthesis, and at the later stages it blocks cytoplasmic trafficking in the new ribonucleoproteins.71 Nucleozin analogs showing antiviral potential against different influenza A species have recently been developed.72
Naproxen: This is a cyclooxygenase 2 inhibitor which has recently revealed anti-influenza activity. It has been shown to form complexes with NP and to competitively inhibit NP binding to RNA. It has in vitro and in vivo activity against influenza A virus.73 Naproxen derivatives with antiviral potential have also been synthesized, and are currently under study.74
NS1 Protein InhibitorsNS1 is a multifunctional protein that binds to mRNA and regulates post-transcription processes: it prevents the activation of the PKR kinase,75,76 and inhibits nuclear export of polyadenylated host mRNA.77–79 NS1 also protects the virus from antiviral cell defense by inhibiting the interferon-mediated response.80–82 Several other drugs that inhibit NS1 activity have also been developed.83 For example, NSC125044 and its derivatives,84 including the compound JJ3297, affect viral replication in vitro, reestablishing the antiviral effect of interferon.85 Baicalin has shown in vitro and in vivo anti-influenza activity by altering the binding domain of the NS1 protein.86
Drugs that Act Against Host ComponentsInsight into the cell functions on which the viral cycle depends has led to the identification of new therapeutic targets in the design of antiviral drugs. Host response modulation is a strategy that minimizes the development of resistant strains, which are not affected by genetic variability in the virus. However, there is a risk of treatment-related side effects. Research into host–influenza interaction has uncovered an extensive network of more than 130 interactions between 10 viral proteins and 87 human proteins.87 More recent analyses have identified 91 host factors that, if inhibited, prevent viral replication in vitro.88 Computerized analysis of drug databases returned hundreds of molecules that interact with host factors necessary for viral replication; most of these are drugs currently in use for other therapeutic purposes.89
Viral Binding and FusionDAS181 (Fludase®): DAS181 is a sialidase catalytic domain/amphiregulin glycosaminoglycan binding sequence fusion protein which cleaves residual sialic acid from the cell surface, preventing the virus from entering the cell. It has shown activity against influenza A and B in animal experiments; however, clinical studies have shown no efficacy in improving influenza symptoms.90,91
Aprotinin: A polypeptide that inhibits host proteases that cleave viral HA. This interferes with the process of viral cell binding and fusion, and anti-influenza activity has been observed both in vitro and in vivo.92,93 Other synthetic compounds that have been developed recently are respiratory epithelium serine protease inhibitors, showing anti-influenza activity in vitro.94
Endocytosis and FusionGlycyrrhizin: This compound has shown anti-influenza A activity by altering the stability of the host cell lipid membrane, preventing endocytosis in enveloped viruses.95–97
LJ001: This molecule has a similar mechanism of action to glycyrrhizin and acts on virus-cell fusion. It has wide-spectrum activity against enveloped viruses, including influenza A.98
Bafilomycin A1 and concanamycin A: Macrolide antibiotics that inhibit vacuolar H+ATPase action that pumps protons from the cell cytoplasm to the interior of the endosome.99,100 More recently, saliphenylhalamide, another vacuolar H+ATPase inhibitor has been studied, showing antiviral efficacy.101
Viral Budding and ReleaseThe enzyme farnesyl diphosphate synthase, which participates in the synthesis of lipid compounds of the plasma membrane, is inhibited by the protein viperin, the expression of which is induced by interferon. This inhibition has been shown to change the composition of the cell plasma membrane, interfering with assembly and budding of viral particles. The enzyme farnesyl diphosphate synthase has been proposed as a potential target for the development of drugs with anti-influenza activity.102
Viral RNA Transcription and TransportThe chaperone protein Hsp90 participates in the nuclear import of viral RNA and in viral polymerase assembly, and has been shown to bind to PB2, with which it is transported into the nucleus, modulating interaction between polymerase basic protein 1 and PA.103 Hsp90 inhibitors, such as geldanamycin and its synthetic derivative 17-AAG, have shown activity against influenza virus in cell cultures.104
Ribavirin and its precursor viramidineare wide-spectrum antivirals. Ribavirin (Virazole®), used in the treatment of hepatitis C, is a guanosine nucleotide analog. Its principal mechanism of action is competitive inhibition of the enzyme inosine 52 monophosphatase dehydrogenase, affecting GTP biosynthesis, and consequently, viral RNA synthesis and viral protein production.105 Despite the positive results obtained in animal studies,106 no efficacy supporting its use has been demonstrated in clinical trials.107,108
Viral Ribonucleoprotein Complex Export and Post-Transcriptional ProcessesVerdinexor (KPT-335): A molecule that inhibits the protein exportin 1, needed for the release of the viral ribonucleoprotein complex from the nucleus to the cytoplasm. It has been shown to possess antiviral activity against several influenza strains.109
Nitazoxanide: An antiparasitic drug that inhibits the maturation of viral HA, and is active against various strains of influenza A virus.110 A randomized clinical study showed a reduction of symptoms in patients with non-complicated influenza infection.111
Intracellular Defense MechanismsThe Raf/MEK/ERK pathway belongs to the family of mitogen-activated protein kinases – MAPK – and is activated by influenza viruses. Blocking this pathway inhibits the nuclear export of ribonucleoprotein due to the interaction with the viral nuclear export protein.112,113In vivo experiments show that administration of U0126, an MEK inhibitor, reduces viral load in lung tissue and improves survival in mice infected with influenza.114 The combination of MEK inhibitors and oseltamivir increases the antiviral efficacy in vitro of the latter.115
The NF-κB transcription factor pathway regulates the expression of antiviral cytokines, and the activation of this pathway has been associated with an increase in influenza virus replication.116,117 Acetylsalicylic acid, which inhibits IKK2, the activator kinase of IκB, has an antiviral effect, modifying the nuclear export of viral ribonucleoprotein.118 Modulation of this pathway is a promising approach for the development of new treatments.
Other Agents with Potential Anti-Influenza ActivityA wide range of drugs used for other diseases have been identified as inhibitors of influenza virus replication in in vitro trials. For example, Na,K-ATPase inhibitors have been shown to possess antiviral activity against different types of DNA and RNA viruses. Cardiac glycosides, specific inhibitors of Na,K-ATPase, have potent in vitro activity against cytomegalovirus and other herpes viruses; ouabain in particular has been seen to inhibit viral protein synthesis.119,120 Ebola virus can also be inhibited in vitro by ouabain121; the anticoronaviral effect of ouabain is mainly a result of its activating the Src kinase intracellular signaling pathway and inhibiting the fusion process.122 Reoviruses are also sensitive to the viral fusion-altering action of ouabain.123 Digoxin, another Na,K-ATPase inhibitor, is active in vitro against the vaccinia virus.124 Different members of the family of cardiac glycosides have recently been found to inhibit influenza A virus replication in vitro.125,126
In summary, the treatment of influenza continues to be a challenge. Current drugs target viral components, so their efficacy may be affected by genetic changes in the influenza virus itself. In recent years, a great number of drugs aimed at modulating host cell response have been investigated and developed. Since these are effective against different viral strains and minimize the emergence of resistant species, these compounds constitute promising new agents in antiviral therapy (Fig. 1).
FundingThis article was funded in part by grants HL-48129 and HL-71643 from the National Institutes of Health.
Conflict of interestThe authors state that they have no conflict of interest.
Please cite this article as: Amarelle L, Lecuona E, Sznajder JI. Tratamiento antigripal: fármacos actualmente utilizados y nuevos agentes en desarrollo. Arch Bronconeumol. 2017;53:19–26.