The clinical presentation of chronic obstructive pulmonary disease (COPD) varies widely, so treatment must be tailored according to the level of risk and phenotype. In 2012, the Spanish COPD Guidelines (GesEPOC) first established pharmacological treatment regimens based on clinical phenotypes. This approach was subsequently adopted by other national guidelines, and since then, have been backed up by new evidence. In this 2017 update, the original severity classification has been replaced by a much simpler risk classification (low or high risk), on the basis of lung function, dyspnea grade, and history of exacerbations, while determination of clinical phenotype is recommended only in high-risk patients. The same clinical phenotypes have been maintained: non-exacerbator, asthma-COPD overlap (ACO), exacerbator with emphysema, and exacerbator with chronic bronchitis. Pharmacological treatment of COPD is based on bronchodilators, the only treatment recommended in low-risk patients. High-risk patients will receive different drugs in addition to bronchodilators, depending on their clinical phenotype. GesEPOC reflects a more individualized approach to COPD treatment, according to patient clinical characteristics and level of risk or complexity.
La enfermedad pulmonar obstructiva crónica (EPOC) presenta una gran heterogeneidad clínica, por lo que su tratamiento se debe individualizar según el nivel de riesgo y el fenotipo. La Guía española de la EPOC (GesEPOC) estableció por primera vez en 2012 unas pautas de tratamiento farmacológico basadas en fenotipos clínicos. Estas pautas han sido adoptadas posteriormente por otras normativas nacionales, y han sido respaldadas por nuevas evidencias publicadas desde entonces. En esta actualización 2017 se ha sustituido la clasificación de gravedad inicial por una clasificación de riesgo mucho más sencilla (bajo o alto riesgo), basándose en la función pulmonar, el grado de disnea y la historia de agudizaciones, y se recomienda la determinación del fenotipo clínico únicamente en pacientes de alto riesgo. Se mantienen los mismos fenotipos clínicos: no agudizador, EPOC-asma (ACO), agudizador con enfisema y agudizador con bronquitis crónica. La base del tratamiento farmacológico de la EPOC es la broncodilatación, y también es el único tratamiento recomendado en pacientes de bajo riesgo. En los pacientes con alto riesgo se añadirán diversos fármacos a los broncodilatadores según el fenotipo clínico. GesEPOC supone una aproximación al tratamiento de la EPOC más individualizado según las características clínicas de los pacientes y su nivel de riesgo o de complejidad.
Chronic obstructive pulmonary disease (COPD) is an underdiagnosed disease with high morbidity and mortality, and is a major public health problem.1
The first Spanish COPD guidelines (GesEPOC) were developed in 2012 as part of the National Health System Quality Plan and the Strategy for COPD of the Ministry for Health, Equality and Social Policy. These were based on an initiative of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR), together with the scientific societies involved in COPD patient care and the Spanish Patient Forum,2 and were the first clinical guidelines on COPD to propose treatment guided by clinical phenotypes, an approach that was subsequently adopted by other national COPD guidelines.3,4 Their implementation has been widespread, as evidenced by an audit conducted in respiratory medicine outpatients in Spain (EPOCONSUL study) between May 2014 and May 2015, which revealed that 46.3% of the medical records of patients with COPD already included phenotype classification according to GesEPOC.5
Diagnostic and treatment guidelines must be periodically updated to keep abreast of ongoing research in COPD and new evidence. This article presents the section on pharmacological treatment of stable COPD in the new GesEPOC 2017. The guidelines have been developed using GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology.6 Details of the protocol, including the PICO questions (Patient, Intervention, Comparison and Outcomes), literature search and evidence tables can be consulted in the complete version of the guidelines7 and in the article's Appendix A. The organisation of GesEPOC is presented in Appendix B.
Initial Care of the Patient With COPDCOPD is defined as a respiratory disease characterized by persistent symptoms and chronic airflow limitation, caused mainly by smoking.
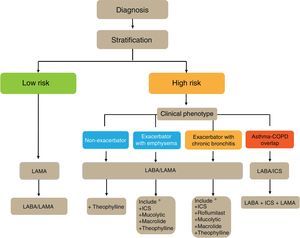
GesEPOC proposes a 4-step patient evaluation process: (1) diagnosis of COPD; (2) stratification into low or high risk; (3) determination of phenotype in high risk patients; and (4) treatment guided by symptoms (low risk) or phenotype (high risk).
DiagnosisThe process starts with diagnostic suspicion in an adult smoker or former smoker of more than 10 pack-years, who presents respiratory symptoms. Spirometry will allow the diagnosis to be confirmed by demonstrating a post-bronchodilator ratio between the forced expiratory volume in the first second (FEV1) and the forced vital capacity (FVC) of less than 0.7. However, it should be noted that this value may underestimate the obstruction in young subjects, and overdiagnose more elderly individuals.8
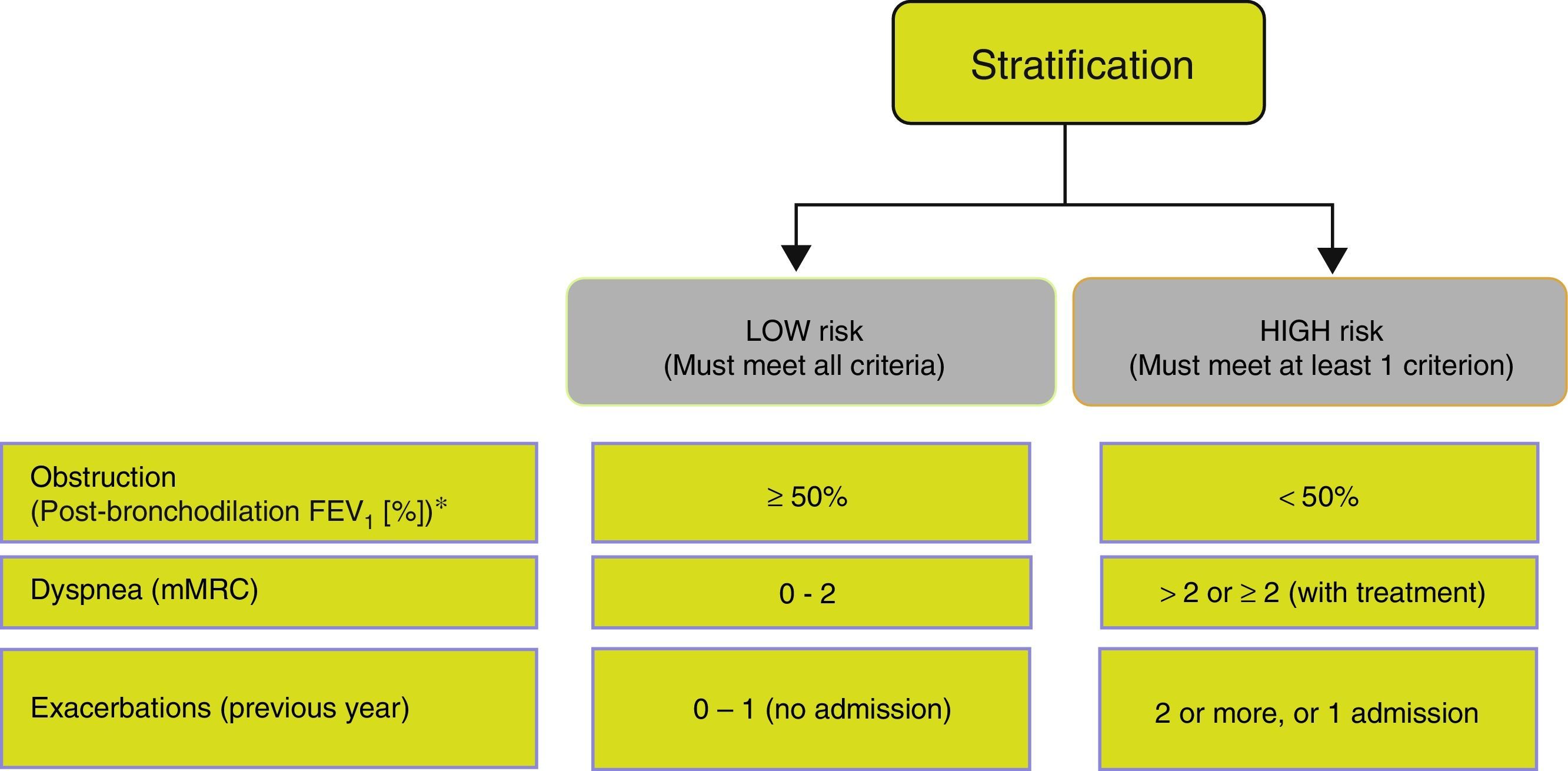
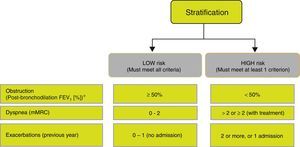
Risk StratificationThe level of risk must then be evaluated. This is defined as the likelihood that the patient will present exacerbations, disease progression, future complications, higher use of healthcare resources or higher mortality. Given the need to simplify risk stratification and adjust intervention levels, both diagnostic and therapeutic, GesEPOC proposes a new classification into 2 risk levels: low and high. This risk classification does not imply referral between healthcare levels.
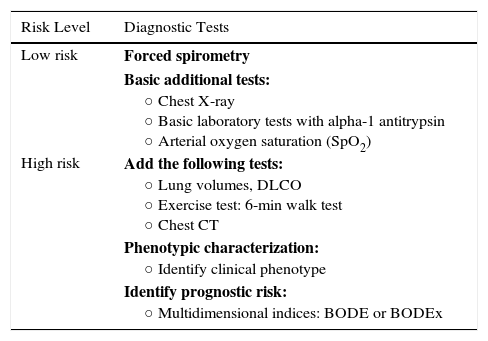
The factors considered in risk evaluation are the degree of obstruction measured by post-bronchodilator FEV1 (%), grade of dyspnea measured by the modified Medical Research Council (mMRC) dyspnea scale, and presence of exacerbations (Fig. 1). The cut-off points established are those recommended by the Global Initiative for Obstructive Lung Disease (GOLD).9 A cut-off point of 50% of the FEV1 has been suggested to differentiate COPD with severe–very severe obstruction from mild–moderate obstruction; dyspnea grade 2 or higher is considered to be a high level of dyspnea in patients on treatment for COPD, while in untreated patients it should be greater than 2; and patients with 2 or more moderate exacerbations (requiring treatment with systemic corticosteroids and/or antibiotics) or with hospital admission due to exacerbation are considered to have a higher risk of exacerbation in the future.9 The components of this risk classification have been shown to be predictive of mortality,10 and are also included in quartile 1 of the multidimensional BODE and BODEx indices, which have a proven ability to predict risk.11,12 The higher the level of risk, the greater the need for diagnostic and/or therapeutic interventions (Tables 1 and 2).
Adaptation of Diagnostic Tests to Risk Levels.
| Risk Level | Diagnostic Tests |
|---|---|
| Low risk | Forced spirometry |
| Basic additional tests: ○ Chest X-ray ○ Basic laboratory tests with alpha-1 antitrypsin ○ Arterial oxygen saturation (SpO2) | |
| High risk | Add the following tests: ○ Lung volumes, DLCO ○ Exercise test: 6-min walk test ○ Chest CT |
| Phenotypic characterization: ○ Identify clinical phenotype | |
| Identify prognostic risk: ○ Multidimensional indices: BODE or BODEx |
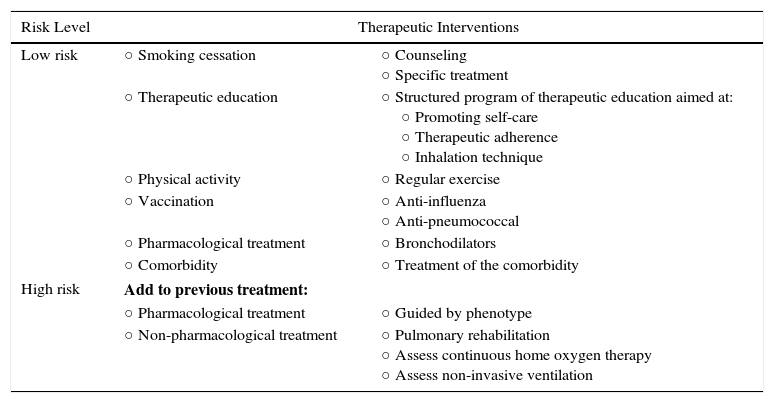
Adaptation of the Care Level to Risk Levels.
| Risk Level | Therapeutic Interventions | |
|---|---|---|
| Low risk | ○ Smoking cessation | ○ Counseling ○ Specific treatment |
| ○ Therapeutic education | ○ Structured program of therapeutic education aimed at: ○ Promoting self-care ○ Therapeutic adherence ○ Inhalation technique | |
| ○ Physical activity | ○ Regular exercise | |
| ○ Vaccination | ○ Anti-influenza ○ Anti-pneumococcal | |
| ○ Pharmacological treatment | ○ Bronchodilators | |
| ○ Comorbidity | ○ Treatment of the comorbidity | |
| High risk | Add to previous treatment: | |
| ○ Pharmacological treatment | ○ Guided by phenotype | |
| ○ Non-pharmacological treatment | ○ Pulmonary rehabilitation ○ Assess continuous home oxygen therapy ○ Assess non-invasive ventilation | |
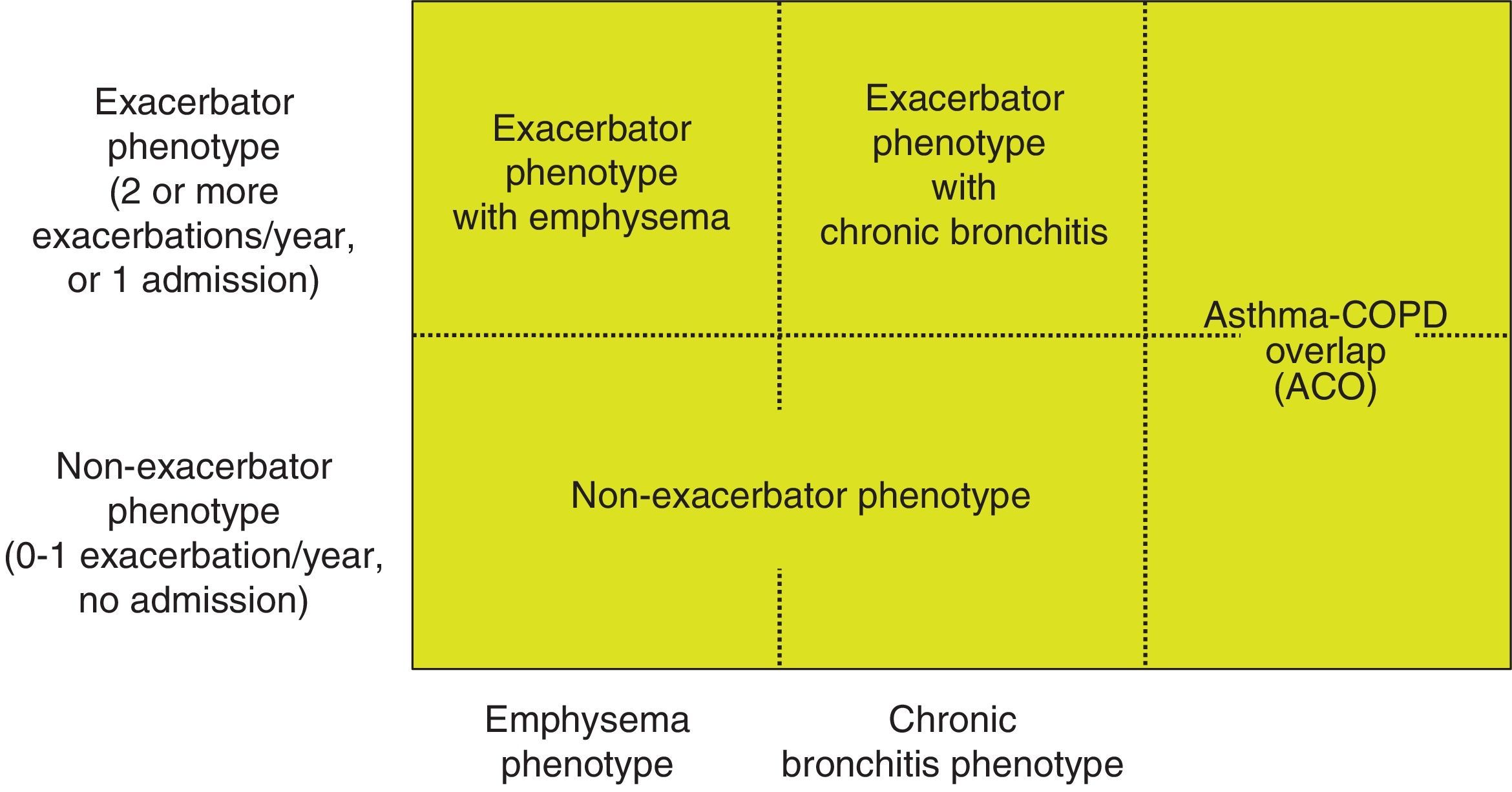
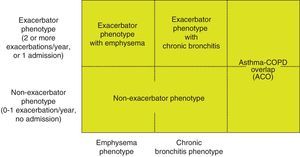
Phenotype, which indicates a different and specific treatment (Fig. 2), must be determined in high risk patients. GesEPOC recognizes 4 phenotypes: (1) non-exacerbator; (2) asthma-COPD overlap (ACO); (3) exacerbator with emphysema; and (4) exacerbator with chronic bronchitis.
The designations of ACO, emphysema and chronic bronchitis are based on the predominant clinical manifestations and fulfillment of the diagnostic criteria. Any of these 3 types of patients can be an exacerbator, so these characteristics are combined to form the 4 clinical phenotypes with different treatment: ACO, exacerbator with chronic bronchitis, exacerbator with emphysema and non-exacerbator.13
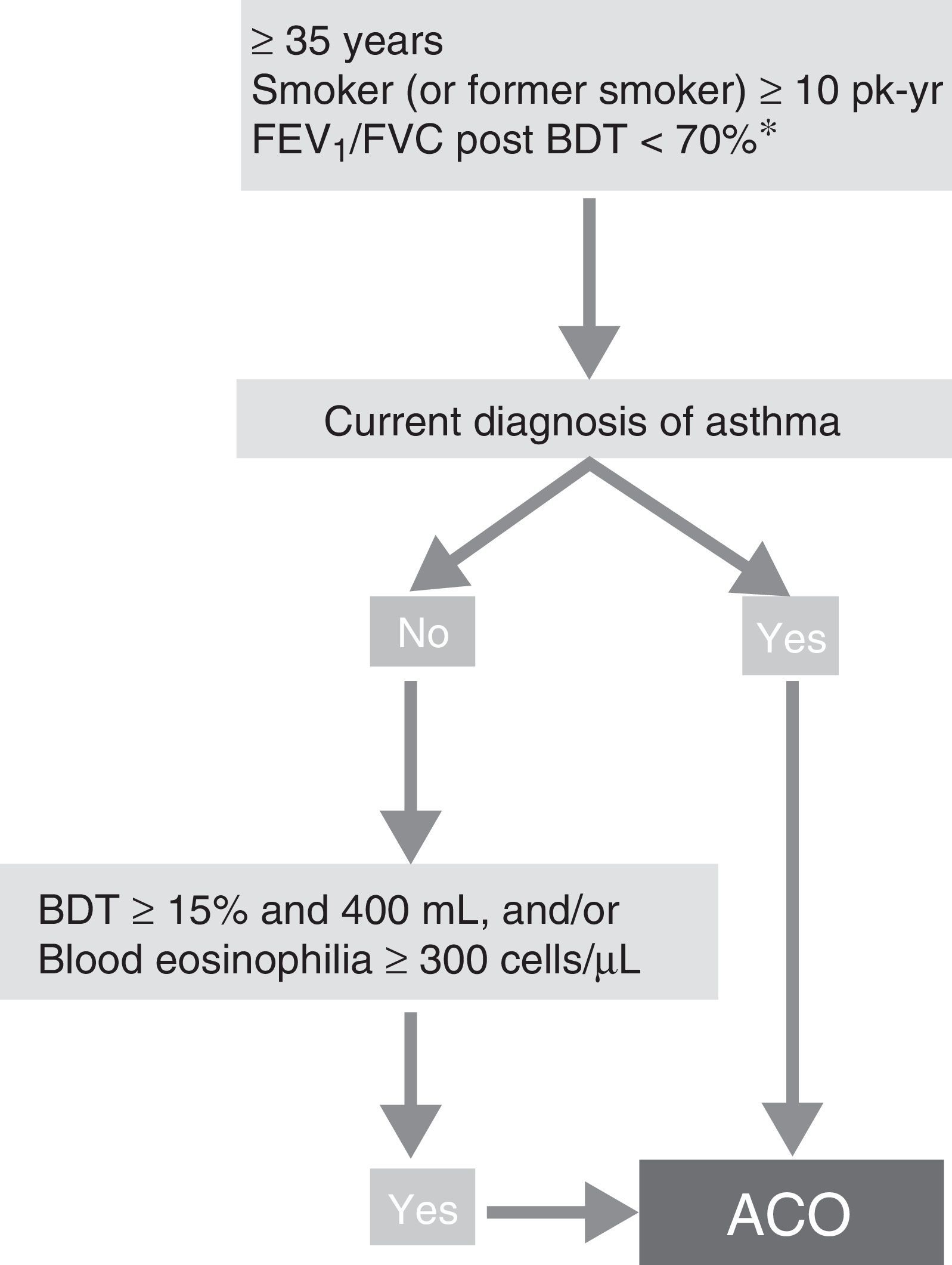
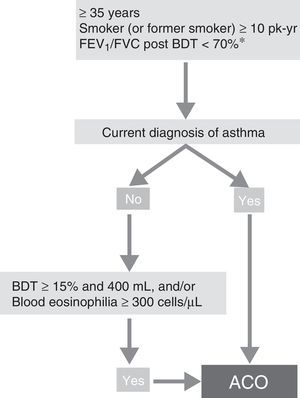
ACO phenotype. According to the recent GesEPOC-GEMA consensus, ACO can be diagnosed in a patient with COPD who also meets the diagnostic criteria for asthma according to current guidelines, or who presents features considered as suggestive of asthma, such as a strongly positive bronchodilator test (increase in FEV1>400mL and 15%) and/or peripheral blood eosinophilia >300 cells per mm3 (Fig. 3).14
Diagnostic algorithm for COPD according to GesEPOC-GEMA (Spanish COPD-Asthma Management guidelines) consensus. * Maintained after treatment with ICS/LABA (6 months). In some cases, also after a cycle of oral glucocorticoids (15 days). ACO – asthma-COPD overlap; BDT – bronchodilator test; ICS – inhaled corticosteroids; LABA – long-acting β2 agonist; Pk-yr – pack-years.
Reproduced with permission of the European Respiratory Society ©: Eur Respir J 2017;49:1700068, doi:10.1183/13993003.00068-2017.
Exacerbator phenotype with emphysema. An exacerbator phenotype is defined as any patient with COPD who presents 2 or more moderate exacerbations in the previous year, defined as those that require at least outpatient treatment with systemic corticosteroids and/or antibiotics, or a severe exacerbation that requires hospital admission.15 To differentiate the new event from therapeutic failure or relapse, these exacerbations must be separated by at least 4 weeks from the resolution of the previous exacerbation, or 6 weeks from its onset in cases where the patient has not received treatment.16 Patients with an exacerbator phenotype have a higher risk of hospitalization, while patients with severe exacerbations have a higher risk of mortality. Due to the different response to pharmacological treatments, it is important to differentiate patients with an emphysematous or chronic bronchitic phenotype.
The emphysema can be better characterized by measurement of gas trapping using static lung volumes and the carbon monoxide diffusing capacity (DLCO) test. Chest computed tomography (CT) will be necessary when considering the possibility of surgical treatment, or if the patient presents frequent exacerbations.17
Exacerbator phenotype with chronic bronchitis. In order to identify chronic bronchitis, the patient must be asked about the presence of cough with expectoration for at least 3 months of the year in 2 consecutive years. In the case of patients with the exacerbator phenotype with chronic bronchitis, high-resolution CT (HRCT) should be carried out to check whether the patient has bronchiectasis18; sputum cultures should also be performed in a stable phase, especially if the sputum is yellowish or dark.19 A repeated positive result suggests that the patient has a chronic bronchial infection.20
Non-exacerbator phenotype. The non-exacerbator phenotype is characterized by presenting a maximum of 1 episode of moderate exacerbation in the previous year. These patients have a lower risk of a deterioration in quality of life (QoL), loss of lung function and mortality than the exacerbator phenotype.
Treatment of Stable COPDThere are 3 general treatment objectives for COPD: to reduce the disease symptoms, to reduce the frequency and severity of exacerbations, and to improve the prognosis. Both short-term benefits (disease control) and mid- to long-term goals (reduction in risk) must be reached.
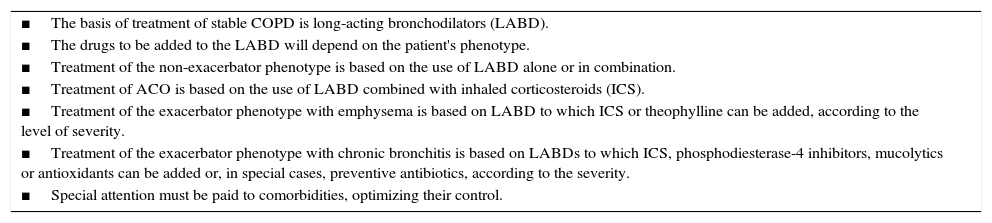
A series of general measures must be taken in any patient with COPD, comprising smoking cessation, proper nutrition, regular physical activity adapted to the patient's age and condition, evaluation and treatment of comorbidities, and vaccination; these will not be addressed in this publication, but are described in greater detail in specific guidelines21,22 and in the complete version of these guidelines.7 The key points in the pharmacological treatment of COPD are shown in Table 3.
Key Points in the Pharmacological Treatment of COPD.
| ■The basis of treatment of stable COPD is long-acting bronchodilators (LABD). |
| ■The drugs to be added to the LABD will depend on the patient's phenotype. |
| ■Treatment of the non-exacerbator phenotype is based on the use of LABD alone or in combination. |
| ■Treatment of ACO is based on the use of LABD combined with inhaled corticosteroids (ICS). |
| ■Treatment of the exacerbator phenotype with emphysema is based on LABD to which ICS or theophylline can be added, according to the level of severity. |
| ■Treatment of the exacerbator phenotype with chronic bronchitis is based on LABDs to which ICS, phosphodiesterase-4 inhibitors, mucolytics or antioxidants can be added or, in special cases, preventive antibiotics, according to the severity. |
| ■Special attention must be paid to comorbidities, optimizing their control. |
The low-risk COPD patient presents mild or moderate airflow obstruction, low grade dyspnea (mMRC<2 without treatment) and non-exacerbator phenotype. No type of anti-inflammatory treatment is indicated in this case, and pharmacological treatment will consist of long-acting bronchodilators (LABD). In the rare event of mild obstruction with few or intermittent symptoms, short-acting bronchodilators (SABD) on demand may be indicated, but the most symptomatic patients should receive LABD continuously.
Short-Acting BronchodilatorsSABD can be of 2 types: anti-cholinergics (SAMA, or short-acting muscarinic antagonists) such as ipratropium bromide, and short-acting beta-2 agonists (SABA) such as salbutamol or terbutaline, and are effective in the rapid control of symptoms. In patients with occasional symptoms, SABD reduce symptoms and improves exercise tolerance.23 These drugs, added to the baseline treatment, are preferred for on-demand treatment of symptoms, regardless of the level of severity of the disease. In patients with persistent symptoms or limitations in their daily activities as a result of their respiratory problem, regular baseline treatment with a LABD will be required.
Long-Acting BronchodilatorsLABD can be beta-2 adrenergics (salmeterol, formoterol, olodaterol, vilanterol and indacaterol – LABA, or long-acting beta-agonists) or anti-cholinergics (tiotropium, aclidinium, glycopyrronium, umeclidinium – LAMA, or long-acting muscarinic antagonists). They should be used as a first step in the treatment of all patients with persistent symptoms who require regular treatment, because they enable better control of symptoms than can be achieved with SABD, and improve both QoL and lung function.24–30 LABD, both LABA and LAMA, have also been shown to reduce the number of exacerbations.30
There are differences between the different LABDs; some have a duration of action of 12h (aclidinium, salmeterol and formoterol) and others 24h (tiotropium, umeclidinium, glycopyrronium, indacaterol, olodaterol and vilanterol). In terms of preventing exacerbations, tiotropium has been shown to be more effective than salmeterol in patients with COPD and a history of at least 1 exacerbation in the previous year.31 Tiotropium has also been shown to be superior to indacaterol in the prevention of exacerbations.32 For this reason, when choosing an LABD as monotherapy, a LAMA is recommended as first choice over a LABA.PICO Question. Which Bronchodilator Should be Recommended as Monotherapy?
Weak recommendation in favor: In patients with COPD who require a long-acting bronchodilator as monotherapy, treatment with a LAMA is recommended.
Specifications: The evidence analyzed is based on greater prevention of exacerbations in studies conducted with tiotropium (a LAMA). In patients with no exacerbations, there are no differences in the clinical efficacy between LAMA and LABA.
LABDs are generally well tolerated, and present few adverse effects. Nevertheless, the following should be considered. LABAs: fine tremor of the extremities, muscle cramps, tachycardia, high blood pressure, peripheral vasodilation, headache, hyperglycemia, hypokalemia, cough, bronchospasm, oropharyngeal irritation and dyspepsia. Treatment with LAMAs may be associated with dry mouth, urinary retention, increased ocular pressure and pharyngeal irritation. It should be noted that clinical trials exclude patients with significant heart disease, so clinicians must be vigilant when using new bronchodilators in these patients.
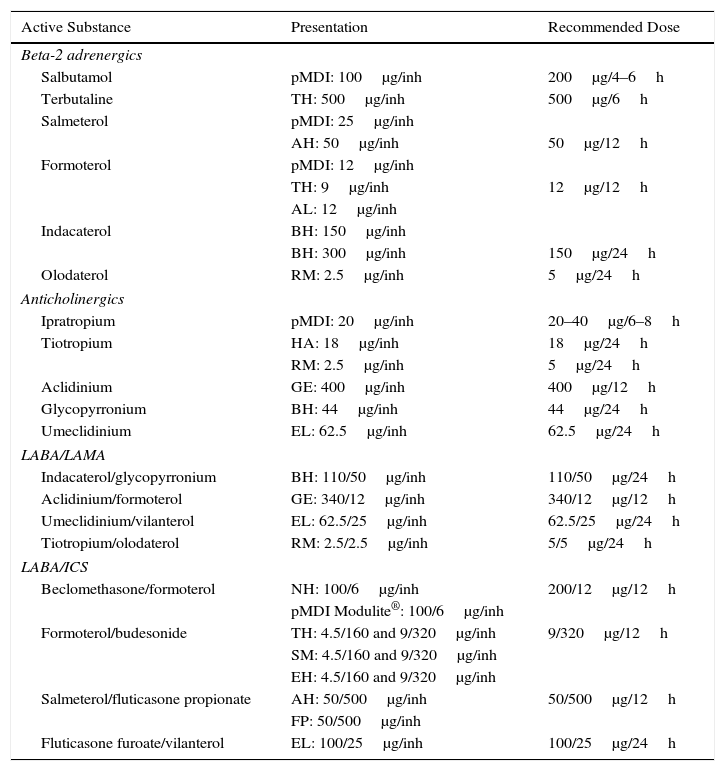
Dual Bronchodilator TherapyIn symptomatic patients or those with clear exercise limitation despite bronchodilator monotherapy, dual bronchodilator therapy should be tried. In these cases, the combination of LABA and LAMA offers an added functional benefit, with less need for rescue medication and improvement in symptoms and QoL compared to monotherapy.33–35 For this reason, in a second treatment step in low-risk patients, two LABDs can be combined to optimize the bronchodilator effect. Existing combinations of LABD (LABA/LAMA) are presented in Table 4.
Characteristics of the Inhaled Drugs for the Treatment of COPD.
| Active Substance | Presentation | Recommended Dose |
|---|---|---|
| Beta-2 adrenergics | ||
| Salbutamol | pMDI: 100μg/inh | 200μg/4–6h |
| Terbutaline | TH: 500μg/inh | 500μg/6h |
| Salmeterol | pMDI: 25μg/inh | |
| AH: 50μg/inh | 50μg/12h | |
| Formoterol | pMDI: 12μg/inh | |
| TH: 9μg/inh | 12μg/12h | |
| AL: 12μg/inh | ||
| Indacaterol | BH: 150μg/inh | |
| BH: 300μg/inh | 150μg/24h | |
| Olodaterol | RM: 2.5μg/inh | 5μg/24h |
| Anticholinergics | ||
| Ipratropium | pMDI: 20μg/inh | 20–40μg/6–8h |
| Tiotropium | HA: 18μg/inh | 18μg/24h |
| RM: 2.5μg/inh | 5μg/24h | |
| Aclidinium | GE: 400μg/inh | 400μg/12h |
| Glycopyrronium | BH: 44μg/inh | 44μg/24h |
| Umeclidinium | EL: 62.5μg/inh | 62.5μg/24h |
| LABA/LAMA | ||
| Indacaterol/glycopyrronium | BH: 110/50μg/inh | 110/50μg/24h |
| Aclidinium/formoterol | GE: 340/12μg/inh | 340/12μg/12h |
| Umeclidinium/vilanterol | EL: 62.5/25μg/inh | 62.5/25μg/24h |
| Tiotropium/olodaterol | RM: 2.5/2.5μg/inh | 5/5μg/24h |
| LABA/ICS | ||
| Beclomethasone/formoterol | NH: 100/6μg/inh | 200/12μg/12h |
| pMDI Modulite®: 100/6μg/inh | ||
| Formoterol/budesonide | TH: 4.5/160 and 9/320μg/inh | 9/320μg/12h |
| SM: 4.5/160 and 9/320μg/inh | ||
| EH: 4.5/160 and 9/320μg/inh | ||
| Salmeterol/fluticasone propionate | AH: 50/500μg/inh | 50/500μg/12h |
| FP: 50/500μg/inh | ||
| Fluticasone furoate/vilanterol | EL: 100/25μg/inh | 100/25μg/24h |
AH – Accuhaler®; AL – Aerolizer®; BH – Breezhaler®; IC – inhaled capsules; EH – Easyhaler; EL – Ellipta®; FP – Forspiro®; GE – Genuair®; HA – Handihaler®; inh – inhalation; LABA/LAMA – long-acting beta-2 adrenergic/long-acting muscarinic antagonist; LABA/ICS – long-acting beta-2 adrenergic/inhaled corticosteroid; NH – Nexthaler®; pMDI – pressurized metered-dose inhaler; p.o. – orally; RM – Respimat®; SM – Spiromax®; TH – Turbuhaler®.
The high-risk COPD patient is defined as a patient who presents severe airflow limitation or high grade dyspnea (mMRC>2 without treatment or 2 or more with treatment) or at least 2 moderate exacerbations or 1 admission in the previous year, or any combination of these factors. These patients require a more detailed diagnostic approach in order to identify their phenotype.36
Dual Bronchodilator TherapyInitial treatment in a patient with high-risk non-exacerbator COPD is dual bronchodilation. This recommendation is based on evidence of superior bronchodilator efficacy compared to monotherapy, accompanied by a significant improvement in dyspnea and QoL, and a reduction in the use of rescue medication. Nevertheless, results obtained in the comparison of exercise capacity between dual bronchodilation and monotherapy are not as consistent.33,34 No direct comparisons have been made to date between the different LABA/LAMA combinations, and indirect comparative analyses do not permit identification of clear differences between them.35,37PICO Question. When Should Dual Bronchodilation (LABA/LAMA) be Used Instead of Single Bronchodilator Therapy?
Weak recommendation in favor: Treatment with dual bronchodilation provides superior bronchodilator efficacy, and is the treatment of choice in symptomatic patients (mMRC≥2) despite treatment with a single bronchodilator.
Specifications: In patients with severe or very severe spirometric impairment, therapy with dual bronchodilation is recommended over monotherapy to begin with, due to its greater effect on lung function.
TheophyllinesTheophyllines, despite being weak bronchodilator drugs, present additive effects to those of common bronchodilators. These drugs have been reported to have a positive effect on diaphragm strength, increased performance of the respiratory muscles, reduction in gas trapping and improvement in mucociliary clearance.38 The usual dose is 200–300mg twice daily in sustained-release tablets. Their limited clinical efficacy and narrow therapeutic margin relegates them to third-line treatment, mainly in high risk patients who continue to be dyspneic following dual bronchodilator therapy.38
The toxicity of theophylline is dose-dependent. When administered on a long-term basis, plasma concentrations should be checked, and clinicians must take into account the risk of interactions with other drugs such as alopurinol, ciprofloxacin, erythromycin, benzodiazepines or cimetidine, among others.
Treatment of the High-Risk Patient With Asthma-COPD Overlap (ACO) PhenotypeThe presence of ACO has been associated with a higher degree of bronchial eosinophilic inflammation, which would account for its greater clinical and spirometric response to inhaled corticosteroids (ICS), and justifies the use of these combined with a LABA as a first option to improve lung function and respiratory symptoms, and reduce exacerbations, if any.39 Follow-up is needed in these cases to evaluate response and, as with asthma, to select the minimum ICS dose necessary for long-term treatment.
An association has been described between eosinophilic inflammation in a stable phase and during exacerbations,40 and ICS are particularly effective in reducing the frequency of exacerbations in patients with COPD and this type of inflammation.41 These exacerbations do not usually present with purulent sputum, but rather with symptoms of rhinitis, wheezing and cough with whitish sputum, and may benefit more from preventive treatment with ICS.40,42
Triple ICS/LABA/LAMA treatment may be required in more severe cases. This triple combination has demonstrated its efficacy in patients with COPD who present great reversibility in the airflow obstruction.43 Tiotropium has also demonstrated efficacy in patients with severe asthma.44
Treatment of the High-Risk Patient With Exacerbator Phenotype With EmphysemaDual Bronchodilator TherapyA randomized clinical trial (RCT) compared dual indacaterol/glycopyrronium vs glycopyrronium with an open-label tiotropium treatment arm. The study population were patients with FEV1<50% predicted, who had had at least 1 exacerbation in the previous year. The results showed a significant 12% reduction in the rate of moderate or severe exacerbations (P=.038) compared to glycopyrronium. There was a numerical reduction compared to tiotropium, but this was not significant.45 This study showed that dual bronchodilation was superior to monotherapy in the prevention of exacerbations in COPD.
A subsequent RCT compared the same combination – indacaterol/glycopyrronium (LABA/LAMA) – vs salmeterol/fluticasone (LABA/ICS) in patients with COPD with FEV1 between 25% and 60% and at least 1 exacerbation in the previous year. The results showed a significant difference in favor of the indacaterol/glycopyrronium combination in the reduction of exacerbations of any degree of severity. Patients taking indacaterol/glycopyrronium also had a lower incidence of pneumonia.46 The results of both studies justify the choice of the combination LABA/LAMA as first-line therapy in the initial treatment of patients with COPD and exacerbator phenotype, except in patients with ACO. It should be remembered that in this latter RCT, patients with a history of asthma and those with blood eosinophilia greater than 600 cells per mm3 were excluded.46
Inhaled CorticosteroidsICS are indicated in patients who present frequent exacerbations despite optimal bronchodilator treatment, because their use combined with a LABA results in a significant decrease in the number of exacerbations and an improvement in QoL.47,48 In COPD, ICS should always be used in combination with a LABD, usually a LABA. The various combinations of LABA/ICS available are presented in Table 4.
Recent sub-analyses of RCTs of LABA/ICS combinations for the prevention of COPD exacerbations have shown that the greatest preventive effect of ICS is achieved in patients with high levels of blood eosinophils.49,50 Furthermore, the risk of pneumonia with the use of ICS in COPD seems to be higher in patients with low eosinophil levels.51 However, these results have not yet been demonstrated in prospective studies designed specifically to that end, nor is there a universally accepted cut-off point for blood eosinophilia to recommend or not the use of ICS in COPD. For this reason, and because registration studies on the LABA/ICS combination did not differentiate between patients with and without eosinophilia, the use of ICS cannot yet be advised against in exacerbator patients with low peripheral blood eosinophil counts, although low efficacy is expected in these cases.42
A balance must be sought between the beneficial effects of ICS and their possible adverse effects. Side effects such as oral thrush, dysphonia, hematomas, reduction in bone mineral density and pneumonia have been observed in large RCTs, although with no increase in mortality.52 Although the risk of pneumonia is greater in patients who take high-dose fluticasone, an increased risk of pneumonia has also been observed associated with the use of budesonide, but of a lesser magnitude.53,54PICO Question. What is the Treatment of Choice in Symptomatic Patients With Exacerbations? LABA/ICS or Dual LABA/LAMA Bronchodilation?
Weak recommendation in favor: In patients who are symptomatic despite a LABD, especially if they present exacerbations, treatment with dual bronchodilation (LABA/LAMA) is preferred over treatment with LABA/ICS.
Specifications: The superior efficacy of LABA/LAMA compared to LABA/ICS in the prevention of exacerbations has been demonstrated with indacaterol/glycopyrronium vs salmeterol/fluticasone.
Triple Therapy (LAMA/LABA/ICS)In high risk patients who do not present good control of exacerbations with 2 drugs (either 2 LABDs or an LABD+ICS), triple therapy with LAMA/LABA/ICS can be used. The few existing studies with triple therapy suggest a greater effect on lung function43 and a decrease in exacerbations and hospital admissions in patients with severe COPD.43,55,56 These effects are evident when a LAMA is added to the LABA/ICS combination,57–59 although greater efficacy when an ICS is added to the LABA/LAMA combination is not clear.55,56 The most important aspect of triple therapy is to determine its efficacy compared to dual bronchodilator therapy (LABA/LAMA).
MucolyticsLong-term use of carbocysteine significantly reduces the number of exacerbations, delays worsening of symptoms and improves QoL in patients with COPD, compared to placebo.60 N-acetylcysteine (NAC) at doses of 600mg daily can reduce the number of exacerbations in patients not treated concomitantly with ICS.61 More recent studies with high-dose NAC (600mg twice daily) have shown a significant reduction in exacerbations, especially in high-risk patients (those with FEV1<50% or with 2 or more exacerbations in the previous year, or both).62,63
Both drugs have an excellent tolerance and safety profile. Although their main mechanism of action suggests that the use of mucolytics should be reserved for exacerbator patients with chronic bronchitis, clinical trials have not selected patients based on the presence of chronic expectoration, so we cannot rule out the possibility that their potential antioxidant action might also prevent exacerbations in patients with exacerbator phenotype with emphysema.PICO Question. When Should Mucolytics be Used to Prevent Exacerbations?
Weak recommendation in favor: In patients with COPD exacerbator phenotype despite adequate treatment, it is suggested that a high-dose mucolytic be added.
Specifications: The costs associated with treatment with mucolytic agents should be discussed with the patient (this treatment is currently not reimbursed in Spain).
Treatment of the High-Risk Patient With Exacerbator Phenotype With Chronic BronchitisThe presence of chronic cough and sputum production is a recognized predisposing factor for exacerbations in COPD. The first treatment step will be dual bronchodilator therapy, and the next step will be to identify the best option for each patient according to their characteristics.64 These options include the drugs already described above, such as ICS and mucolytics, as well as phosphodiesterase-4 inhibitors and long-term antibiotics.
Phosphodiesterase-4 InhibitorsRoflumilast is an oral anti-inflammatory drug that acts by selective inhibition of phosphodiesterase-4. It has been shown to prevent exacerbations in patients with severe COPD who present chronic cough and sputum production, and also suffer frequent exacerbations.65 This effect is maintained when roflumilast is added to maintenance treatment with an LABD, either LABA or LAMA. It also achieves a significant increase in trough FEV1 (between 50 and 70mL on top of that achieved with salmeterol or tiotropium).65,66 The effect of roflumilast in the prevention of exacerbations has been observed even when added to triple therapy (LABA/LAMA/ICS).67 A recent study has shown that this medication is more effective in reducing exacerbations in patients with more severe COPD who require hospital admission.68 The usual dose is 500μg orally once daily.
Adverse effects with roflumilast usually appear at the start of treatment, are rapidly detected by the patient and usually disappear within the first 4 weeks, although they may occasionally lead to discontinuation of the drug. The most common adverse effects are weight loss, gastrointestinal disturbances, nausea, headache and loss of appetite. The safety profile of roflumilast is not modified by any concomitant treatment that the patient may be taking for their COPD. The use of roflumilast with theophyllines should be avoided.PICO Question. When Should Roflumilast be Used to Prevent Exacerbations?
Weak recommendation in favor: Roflumilast has been suggested as a second-line drug to prevent exacerbations in patients with the exacerbator phenotype with chronic bronchitis and severe airflow limitation.
Specifications: Due to its safety profile, tolerance to the drug may be poor, and physicians should aware of the possible onset of adverse effects.
Use of Antibiotics in Stable COPDLong-term treatment with macrolides is indicated in high-risk patients with at least 3 exacerbations in the previous year despite adequate inhaled therapy.69,70
Macrolides administered on a long term basis have been shown to significantly reduce the number of exacerbations in stable patients with severe COPD.71 The regimens used were: erythromycin 250mg twice daily for 1 year,72 azithromycin 500mg daily for 3 days a week for 1 year,69,73 and azithromycin 250mg daily for 1 year.74 The results of all these studies have consistently shown a significant reduction in exacerbations. However, the study populations and regimens were different, so it is difficult to make a recommendation. It should be noted that in the study by Albert et al.,74 increased bacterial resistance to macrolides and an increase in hearing problems were found in patients treated with azithromycin. Due to its similarity to regimens used in patients with bronchiectasis, the recommended dose is azithromycin 250 or 500mg daily for 3 days a week for 1 year, repeated in successive winter periods (from November to May) if response is positive (absence of exacerbations). This treatment should be reserved for reference centers with clinical, auditory, liver biochemistry and microbiological monitoring, with identification of microorganisms in sputum and antibiotic susceptibility testing.70 There is no evidence of the efficacy of this treatment beyond 1 year of follow-up, so the possible risk-benefit should be evaluated annually.70PICO Question. When Should Long-Term Macrolides be Used to Prevent Exacerbations?
Weak recommendation in favor: In patients with COPD with an exacerbator phenotype, with at least 3 exacerbations in the previous year despite adequate treatment, long-term treatment with macrolides is recommended.
Specifications: The authors believe that this indication should be restricted to patients with frequent exacerbations and a severe degree of obstruction. Once selected, possible treatment-associated adverse effects such as prolongation of the QT interval, hearing loss or generation of resistances should be strictly monitored.
The use of quinolones during periods of stability has been shown to eradicate the bacteria present in the sputum in most patients with severe COPD and frequent exacerbations.75 One RCT evaluated the efficacy of cyclical administration of moxifloxacin (400mg daily for 5 days every 2 months) for 1 year in patients with stable COPD.76 The results showed that the treatment significantly reduced 45% of exacerbations in patients who presented purulent or mucopurulent sputum, i.e. those with a higher probability of suffering a chronic bacterial bronchial infection.
Since quinolones are the preferred therapy in the treatment of exacerbations in patients with severe COPD, it is important to preserve this class of antibiotics, so the long-term use of quinolones with a preventive objective is not recommended.PICO Question. When Should Long-Term Quinolones be Used to Prevent Exacerbations?
Strong recommendation against: In patients with COPD in a stable phase, administering chronic treatment with fluoroquinolones in order to prevent exacerbations is not recommended.
Specifications: The group drafting the guidelines agrees with rationalizing the use of fluoroquinolones, and that they should not be indicated as a preventive regimen for exacerbations due to the risk of development of bacterial resistance.
Patients who are candidates for long-term antibiotic treatment are those with a high likelihood of having bronchiectasis. Bronchiectases can help perpetuate a vicious circle, amplifying the underlying inflammation and inducing the presence of frequent exacerbations,18,77 and are even associated with higher mortality.78 In patients with COPD and bronchiectasis, the infectious component should be treated according to bronchiectasis treatment guidelines.79
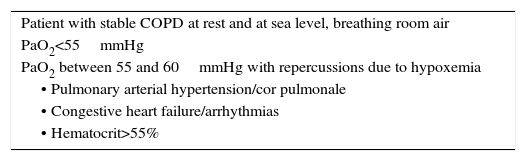
Other Specific TreatmentsLong-Term Home Oxygen TherapyLong-term home oxygen therapy (LTOT) is a treatment that improves survival in patients with COPD and respiratory failure.80 It has also been shown to reduce the number of exacerbations and hospitalizations, and to improve exercise capacity and QoL.81 The benefits achieved with oxygen therapy depend on the duration of treatment. LTOT should be given for at least 16h daily, in order to maintain a resting PaO2≥60mmHg or oxygen saturation ≥90% at sea level. The therapy is indicated when the disease is in a stable phase, and should be based on arterial blood gases (Table 5).
Indications for Continuous Home Oxygen Therapy.
| Patient with stable COPD at rest and at sea level, breathing room air |
| PaO2<55mmHg |
| PaO2 between 55 and 60mmHg with repercussions due to hypoxemia |
| • Pulmonary arterial hypertension/cor pulmonale |
| • Congestive heart failure/arrhythmias |
| • Hematocrit>55% |
The use of LTOT in patients with intermittent hypoxemia, such as the hypoxemia that appears during sleep, or in patients with mild to moderate hypoxemia at rest or during exercise, has failed to show effects on survival or time to first hospital admission or other clinical parameters, so it is not recommended.82 In the absence of criteria for LTOT, nocturnal oxygen therapy can be considered in patients with proven nocturnal oxyhemoglobin desaturation (<90% for at least 30% of the total recording time) and sequelae related with the hypoxia (polycythemia or signs of right-sided heart failure). Continuous positive airway pressure (CPAP) or mechanical ventilation should be considered,83 which can replace or complement the oxygen therapy, but sleep apnea syndrome must be ruled out.
Portable oxygen therapy improves the ability of patients with severe COPD to exercise. It can be used in cases of limitation due to dyspnea and with desaturations of less than 88% in the walk test.84 The improvement obtained in both the dyspnea and the distance walked should be re-evaluated in the first 2 months.84 Nevertheless, further studies are required to define the benefits.
Alpha-1 Antitrypsin Augmentation TherapyAugmentation treatment with purified alpha-1 antitrypsin (AAT) is recommended by the leading scientific societies (American Thoracic Society, European Respiratory Society and SEPAR)85,86 in patients with pulmonary emphysema with severe AAT deficiency and homozygous PiZZ phenotype or rare deficiency variants, due to their effect in slowing the loss of lung density measured by HRCT. The RAPID study is the largest RCT conducted to date to evaluate the efficacy of augmentation treatment in slowing the progress of emphysema in patients with severe AAT deficiency.87 The results showed that after 2 years of follow-up, lung density loss measured by HRCT was significantly less in patients on treatment vs patients in the placebo group. Moreover, the study had an open-label extension phase for a further 2 years, in which patients who had been on placebo received active treatment. The authors observed that active treatment significantly slowed disease progression in relation to the previous 2-year period with placebo. Nevertheless, the excess lung density loss that they suffered during the 2 years with placebo did not recover. This suggests the importance of early identification and treatment of patients at risk of rapid disease progression to prevent lung damage.88 Their inclusion and exclusion criteria are well defined in the guidelines specified.85,86
Serum alpha-1 antitrypsin levels should be measured at least once in all patients with COPD. After identifying a patient with AAT deficiency, a family study should be carried out to identify possible undiagnosed cases.85,86 Any cases detected must be reported to the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency,89 and they should be referred to a reference center for complete diagnosis and assessment for possible augmentation therapy and a family study.
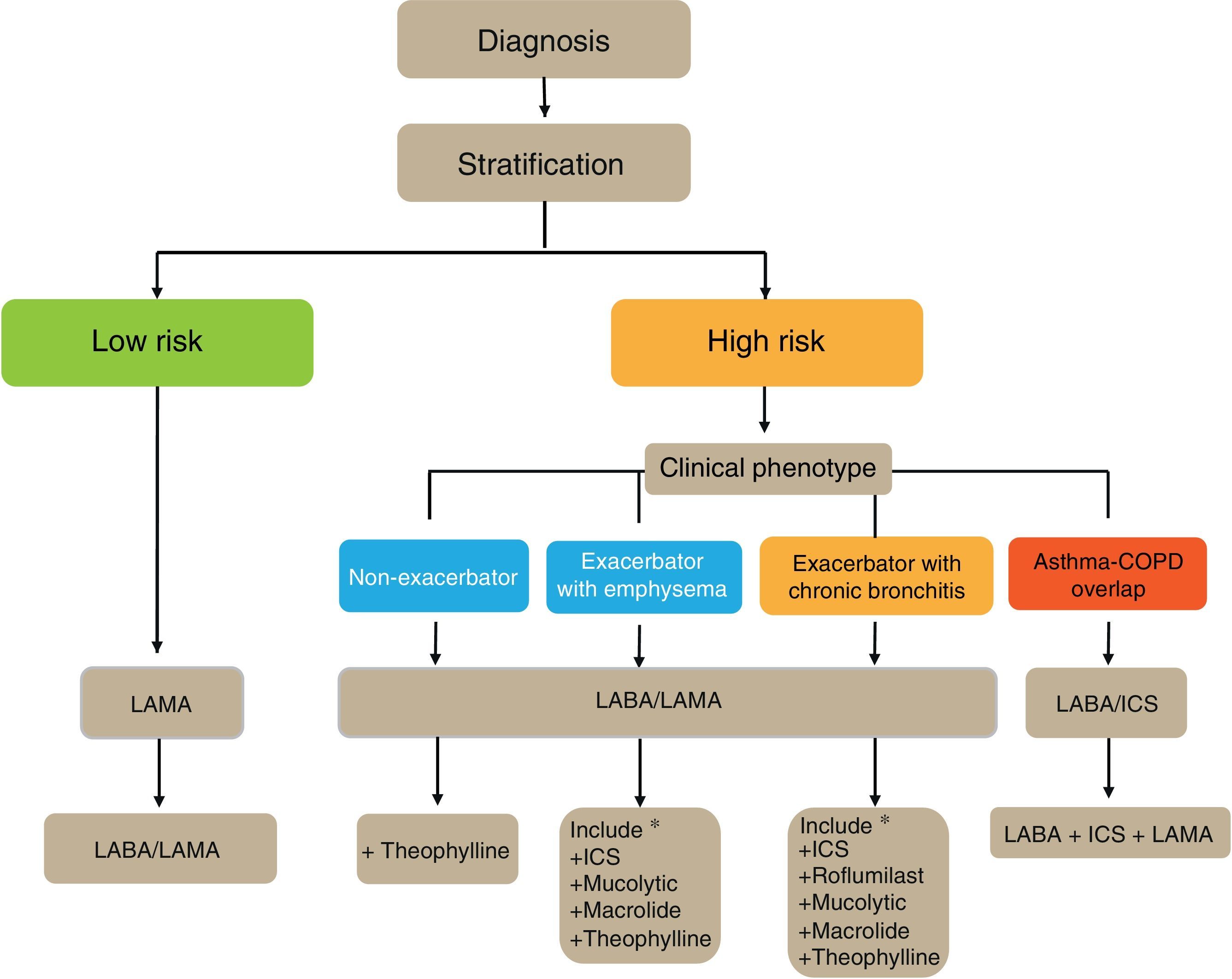
Flow Chart of the Initial Treatment of Stable COPDThe flow chart of the initial treatment of stable COPD is presented in Fig. 4. Low-risk patients initially receive a LAMA. In cases of persistence or worsening of symptoms or exacerbations, treatment is increased to dual bronchodilator therapy.
High-risk patients receive first-line treatment with a LABA/LAMA combination, except in cases of ACO, in which the LABA/ICS combination is indicated. The phenotype should be determined in these patients, and in the case of insufficient control, new drugs should be added in accordance with the patient's phenotype. Before increasing the medication, it is important to investigate adequate adherence, correct inhalation technique and also to check whether there are any comorbidities that may be causing the symptoms.
Adaptation of Treatment During Follow-UpThe increase in treatment in accordance with progression of the disease or its severity, and in particular due to persistence or worsening of symptoms or the presence of exacerbations, is well established. However, there is very little evidence on the possible reduction or withdrawal of treatment in patients who achieve improvement or clinical stability. The following suggestions are based on the existing evidence:
- (a)
Bronchodilator treatment exerts its effect only during administration, so it is very likely that withdrawing a bronchodilator or replacing it with another with lower bronchodilatory potency or shorter duration of action will cause functional and/or symptomatic worsening.90
- (b)
Tapering the ICS dose until the minimum effective dose is reached can be tried in patients with ACO, as is done in asthma. It is not recommended to leave these patients on LABD treatment without ICS.
- (c)
In patients with the exacerbator phenotype, a tapered regimen cannot be specified in the case of stability. Treatment must be tapered based on clinical judgment, starting with the withdrawal of drugs that are probably least active or that present a higher likelihood of short- or long-term adverse effects.
- (d)
Long-term ICS treatment is associated with the possible onset of adverse effects.54 For this reason, it is essential to evaluate the risk-benefit ratio of using these drugs in each patient. Although there is a high degree of consensus that withdrawal of ICs is possible, there is less agreement when it comes to defining the characteristics of patients who are candidates for withdrawal.91
Some studies suggest that indiscriminate withdrawal of ICS may increase bronchial inflammation,92 and can be associated with an increased risk of exacerbations.93 However, recent studies including large populations of patients with moderate or severe COPD have failed to find a higher risk of exacerbations when ICS are withdrawn, provided that they are replaced with adequate bronchodilation.94,95 Post hoc analyses of these studies show that the blood eosinophil count may also be a good marker to identify patients who are candidates for risk-free ICS withdrawal, even in the presence of severe airflow obstruction and exacerbations in the previous year, provided they continue treatment with dual bronchodilator therapy.96 Similarly, other studies suggest that ICS withdrawal is safe in mild-moderate COPD patients (FEV1>50%) who do not present exacerbations or even with exacerbations in the previous year, provided that the bronchodilation is optimized.97
For practical purposes, ICS withdrawal is recommended in all patients receiving them out of the approved indication. Withdrawal should also be considered in more severe patients (FEV1<50%) if they have a risk of ICS-related adverse effects: previous pneumonia, osteoporosis, poorly controlled diabetes, fragile skin, etc. If they remain stable, with no exacerbations in the previous year, blood eosinophils should be assessed and withdrawal recommended if the count is less than 300cells/mm,3 as higher counts constitute a criterion for ACO and need for the use of ICS.
Although the largest clinical study conducted to date used a tapering ICS dose regimen,94 the evidence accumulated in other clinical studies suggests that withdrawal can be tried without reducing the dose.97,98
Pharmacological treatment of patients with COPD should be re-evaluated periodically, since age, changes in comorbidities, seasonality of symptoms and exacerbations, availability of new drugs and new treatment strategies, together with hygiene and non-pharmacological measures, should allow treatment to be personalized and clinical outcomes improved.
Conflict of InterestMarc Miravitlles has received honoraria for scientific advice and/or for lecturing from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grupo Ferrer, GlaxoSmithKline, Grifols, Laboratorios Esteve, Teva, Cipla, Menarini, Novartis and Gebro Pharma.
Borja G. Cosío has received honoraria for scientific advice and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Teva, Menarini, and Novartis.
Juan José Soler-Cataluña has received honoraria for scientific advice and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, Gebro, Grupo Ferrer, GlaxoSmithKline, Laboratorios Esteve, Teva, Menarini, Novartis and Pfizer.
Myriam Calle has received honoraria for lecturing from Novartis, AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim.
Pere Almagro has received honoraria for scientific advice and/or for lecturing from Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Laboratorios Esteve, Menarini, and Novartis.
Ciro Casanova Macario has received honoraria for lecturing and/or scientific advice in the last 3 years from Astra-Zéneca, Boehringer-Ingelheim, Gebropharma, GlaxoSmithKline, Laboratorios Esteve, Menarini, Novartis and Rovi.
Jesús Molina has received honoraria for scientific advice and/or for lecturing from Astra-Zéneca, Boehringer Ingelheim, Chiesi, Gebro, GlaxoSmithKline, Laboratorios Esteve, Menarini, Mundipharma, Novartis, Pfizer, Rovi and Teva.
Pere Simonet has received honoraria for lecturing from Boehringer Ingelheim, Menarini, Mundipharma, GlaxoSmithKline and Teva.
José Antonio Quintano has received honoraria for scientific advice and/or for lecturing from AstraZeneca, Boehringer-Ingelheim, Esteve, Gebro, Grifols, Menarini, Mundipharma, Pfizer, ROVI and TEVA.
Julio Ancochea has received honoraria for scientific advice and/or for lecturing from Actelion, Air Liquide, Almirall, AstraZeneca, Boehringer Ingelheim, Carburos Médica, Chiesi, Faes Farma, Ferrer, GlaxoSmithKline, InterMune, Linde Healthcare, Menarini, MSD, Mundipharma, Novartis, Pfizer, Roche, Rovi, Sandoz, Takeda and Teva.
Coordinator: Marc Miravitlles, Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Members of the working group: Myriam Calle, Borja García-Cosío, Juan Antonio Riesco, Eusebi Chiner, Ciro Casanova and Juan José Soler-Cataluña (SEPAR). Joan B. Soriano (SEPAR-epidemiology). Julio Ancochea, scientific coordinator, COPD Strategy of the National Health Service (SEPAR). Pere Almagro, Spanish Society of Internal Medicine (SEMI). Elena Gimeno (SEPAR-Physiotherapy). Eulogio Pleguezuelos, Spanish Society of Rehabilitation and Physical Medicine and Spanish Society of Cardio-Respiratory Rehabilitation (SERMEF/SORECAR). José Antonio Quintano, Spanish Society of Primary Care Physicians (SEMERGEN). Juan Antonio Trigueros, Spanish Society of General and Family Medicine (SEMG). Jesús Molina and Miguel Ángel Lobo Álvarez, Spanish Society of Family and Community Medicine (semFYC). Pere Simonet, Society of Respiratory Medicine in Primary Care (GRAP). Pascual Piñera and Adolfo Simón, Spanish Society of Emergency Medicine (SEMES). Carme Hernández and Carmen Mata (SEPAR-Nursing). Leopoldo Palacios and Carlos Verdejo, Spanish Society of Geriatrics and Gerontology (SEGG). María Emilia Carretero Díaz, Spanish Patient Forum (FEP). David Rigau and Ena Pery Niño de Guzman Quispe, Iberoamerican Cochrane Centre.
Please cite this article as: Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Guía española de la EPOC (GesEPOC) 2017. Tratamiento farmacológico de la EPOC estable. Arch Bronconeumol. 2017;53:324–335.