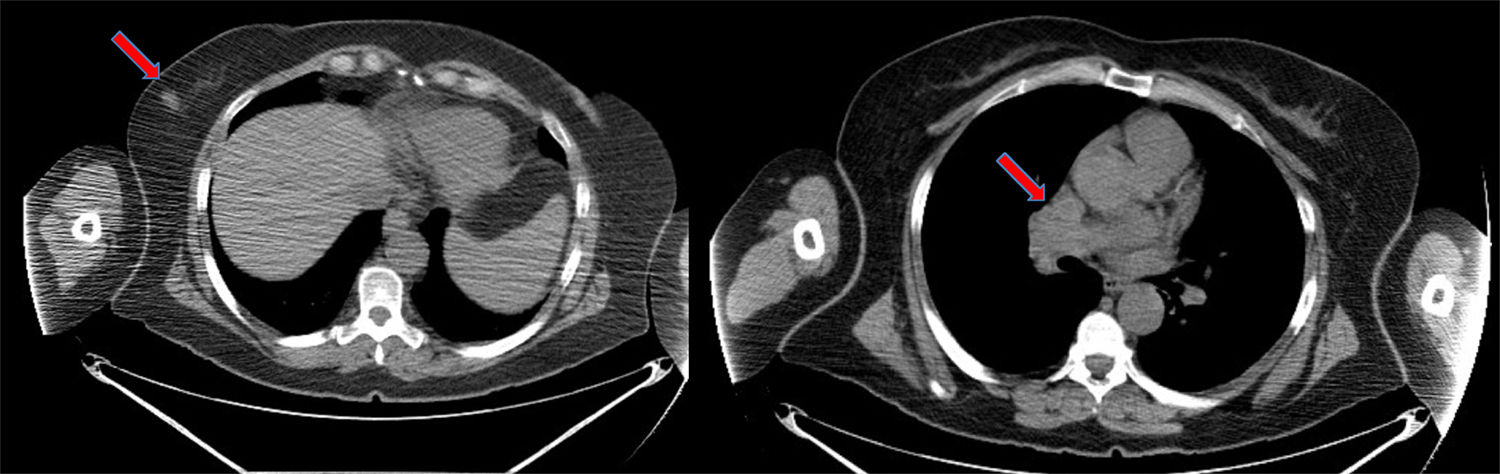
We report the case of a 58-year-old patient with a significant history of smoking and previous pituitary microadenoma. In August 2014, she consulted due to the appearance of a hard, rapidly growing mass in her right breast detected by self-examination. A screening mammogram 6 months previously had been negative. She underwent mammogram/ultrasound, which showed a dense 18-mm nodule with undefined, spiculated borders in the lower external quadrant, BI-RADS category 5, with no axillary lymphadenopathies. Biopsy of the nodule showed a high-grade neuroendocrine carcinoma with no expression of hormone receptors or HER2 onco-protein, and a cell proliferation index (Ki67) greater than 80%. Given the presence of a rare, highly undifferentiated tumor, we decided to complete the study with imaging tests to rule out distant disease and confirm the mammary origin of the lesion. Computed tomography (CT) confirmed the presence of the breast nodule and showed mediastinal lymphadenopathies in the pathological range (Fig. 1), and multiple millimetric pulmonary nodules, septal thickening, and nodules suggestive of carcinomatous lymphangitis. These findings were confirmed on positron emission tomography, and other disease foci were ruled out. Endobronchial ultrasound was performed, with aspiration of the mediastinal lymphadenopathies, which again showed the presence of a high-grade TTF1-negative neuroendocrine carcinoma with a Ki67 of 98%.
In view of the unusual nature of the case, it was discussed in the multidisciplinary session, and the specimens obtained from both sites were compared by the pathology lab. Similar morphology was observed: small and medium-sized cells with minimal cytoplasm, rounded nuclei with glandular chromatin and occasional nucleoli, arranged in the form of solid nests, with no glandular or squamous identification. The immunohistochemical profile showed expression in both cases of cytokeratin 19, synaptophysin, and chromogranin. After review of the various tests and the peculiarity of the case, with the presence of synchronous high-grade neuroendocrine tumors, 2 possible scenarios were considered: the presence of primary lung cancer with secondary breast involvement or a primary breast tumor with lung involvement. The first situation was ruled out when no lung mass was observed in the imaging tests, whereas there was clear evidence of the mammary nodule. Moreover, TTF1 negativity in the specimens supported this hypothesis, leading us to think that the most likely scenario was primary breast tumor with secondary pulmonary metastases. With this in mind, we decided to start polychemotherapy for metastatic disease based on a platinum-etoposide doublet, and a total of 6 cycles were administered. In a post-treatment follow-up with CT and PET-CT, complete morphological and metabolic response was observed, both in the breast and in the lymph nodes. Radiation therapy was subsequently given to the breast and the mediastinum, followed by prophylactic cranial irradiation. However, despite the good initial response, cerebral and systemic recurrence were observed 4 months later. The patient did not respond to the different treatment regimens, and died soon after.
Breast cancer is the most common malignant tumor worldwide, and the most predominant histopathological variety is infiltrating ductal carcinoma.1 One unusual variety is neuroendocrine carcinoma, described in 1977 to refer to a group of neoplasms that, while not demonstrating neuroendocrine-secreting granules, may come from argyrophilic cells originating in the neural crest that might have migrated to the mammary ducts. This histology was included in the WHO classification in 2003,2 and 3 variants are distinguished: solid, small cell and large cell. These tumors can be low and high grade which, like hormone receptor and HER2/neu onco-protein expression, has a prognostic and predictive impact. The presence of a small cell component generally determines the biologic aggressivity of the tumor. Immunohistochemistry is important for diagnosis, and includes specific markers such as cytokeratin, neuron-specific enolase, synaptophysin, serotonin, bombesin, and chromogranin, among others.3 In our case, these techniques showed the tumor was positive for synaptophysin and chromogranin, and negative for breast tumor markers (estrogen and progesterone hormone markers, and c-erb-2).
As the natural history of this tumor is similar to that of other equivalent lung masses that have a tendency toward early metastasis, the therapeutic approach is also similar, given the lack of prospective clinical trials in this very special scenario.4 In the case of extensive disease, the only possible treatment modality is palliative chemotherapy, perhaps with the addition of radiation therapy on an individualized basis, as received by our patient. In extrapulmonary neuroendocrine tumors, cerebral involvement is less common, so brain irradiation has less potential benefit.5 Prognosis in extrapulmonary tumors differs according to the series, as some authors show it to be similar or even better than in small cell lung cancer, while others claim otherwise. However, according to the available data, mean survival in limited and extended disease varies between 1.4 and 3.5 years, and 8 and 12 months, respectively.
In our case, the patient had an aggressive, high-grade tumor, as seen from its high proliferation rate, the absence of hormone receptor and HER2 expression, its rapid growth (this was an interval cancer, since the mammogram performed 6 months before diagnosis was normal), and the presence of mediastinal lymphadenopathies at the time of diagnosis. These data were markers for poor disease prognosis. Since the disease had already spread, we opted for the administration of palliative CT, to which it is particularly sensitive. Despite the excellent response with an absence of radiologically visible macroscopic disease, and consolidation with sequential and prophylactic holocranial RT, the patient developed multisystem progression shortly afterwards, determining a poor short-term prognosis.
Please cite this article as: Cruz Castellanos P, Quintana L, de Castro J. Carcinoma microcítico de mama con afectación pulmonar. Arch Bronconeumol. 2018;54:586–587.