International guidelines recommend the use of nocturnal long-term non-invasive ventilation (NIV) based on clinical and gasometrical criteria,1 primarily to control PaCO2 and reduce mortality. In France, >70,000 patients are dependent on NIV. In addition to alveolar hypoventilation, these patients often experience sleep-related symptoms.2
Numerous studies have investigated whether NIV enhances sleep quality. Patients with restrictive respiratory insufficiency demonstrated positive changes in polysomnographic sleep parameters,3 whereas those with chronic obstructive pulmonary disease (COPD) experienced inconsistent benefits.4 Additionally, patients with obesity hypoventilation syndrome using NIV had higher rates of slow-wave and rapid eye movement sleep. However, these improvements did not significantly impact the Pittsburgh Sleep Quality Index (PSQI).5
NIV improves sleep parameters without fully restoring sleep quality, potentially due to suboptimal treatment. In addition to preventing hypoventilation, several NIV parameters should be considered to optimize treatment. Monitoring the quality of nocturnal ventilation presents challenges and mainly relies on polysomnography.6 Recently, the SomnoNIV group proposed that ventilation efficiency should be monitored based on oxygen and carbon dioxide levels, compliance, leakage, and the Apnea-Hypopnea Index (AHI).7
We hypothesized that, if the aforementioned criteria are unfulfilled,7 NIV patients may experience poor sleep quality, as assessed by the PSQI. Therefore, we evaluated the sleep quality of our NIV patients, factors associated with poor sleep quality, and potential correlations with the SomnoNIV ventilation criteria.
This prospective observational study included consecutive adult patients who were admitted to the Respiratory Diseases Service of Bordeaux University Hospital for NIV between July 2, 2022 and May 26, 2023. We excluded patients who received NIV for <3 months, were non-compliant, had an acute medical event within the preceding 7 days, or discontinued NIV after discharge from the hospital. We recorded age, sex, anthropometric data, smoking status, medical history, pulmonary function test results, medications, and details of NIV initiation. Alveolar hypoventilation was assessed using arterial blood gas analysis performed during hospitalization and nocturnal transcutaneous capnography performed at home a few days before the follow-up visit. NIV quality was assessed at each visit, with a focus on primarily controlling leaks and residual obstructive events. For the patients without abnormal leaks (>24L/min) and residual obstruction, an analysis of the patient–ventilator interaction through the flow curves of the built-in software was done by two different pneumologists. If there was a discordance, a third pneumologist would make another analysis. A patient-ventilator asynchrony (PVA) was considered clinically relevant only if a simple modification of the NIV parameters was done during the assessment or if a polygraphy was indicated to further investigate. This framework of analysis has been described elsewhere.8,9 Sleep quality was evaluated using the PSQI and the Epworth Sleepiness Scale. Good sleepers had PSQI scores >5. Univariate analyses were conducted to identify risk factors of poor sleep. Linear regression analyses were used to determine correlations between these parameters and PSQI scores.
All the patients gave their informed consent to participate in the study, and it was approved by the Research Ethics Committee of the University Hospital of Bordeaux (reference: CERBDX 2023-143).
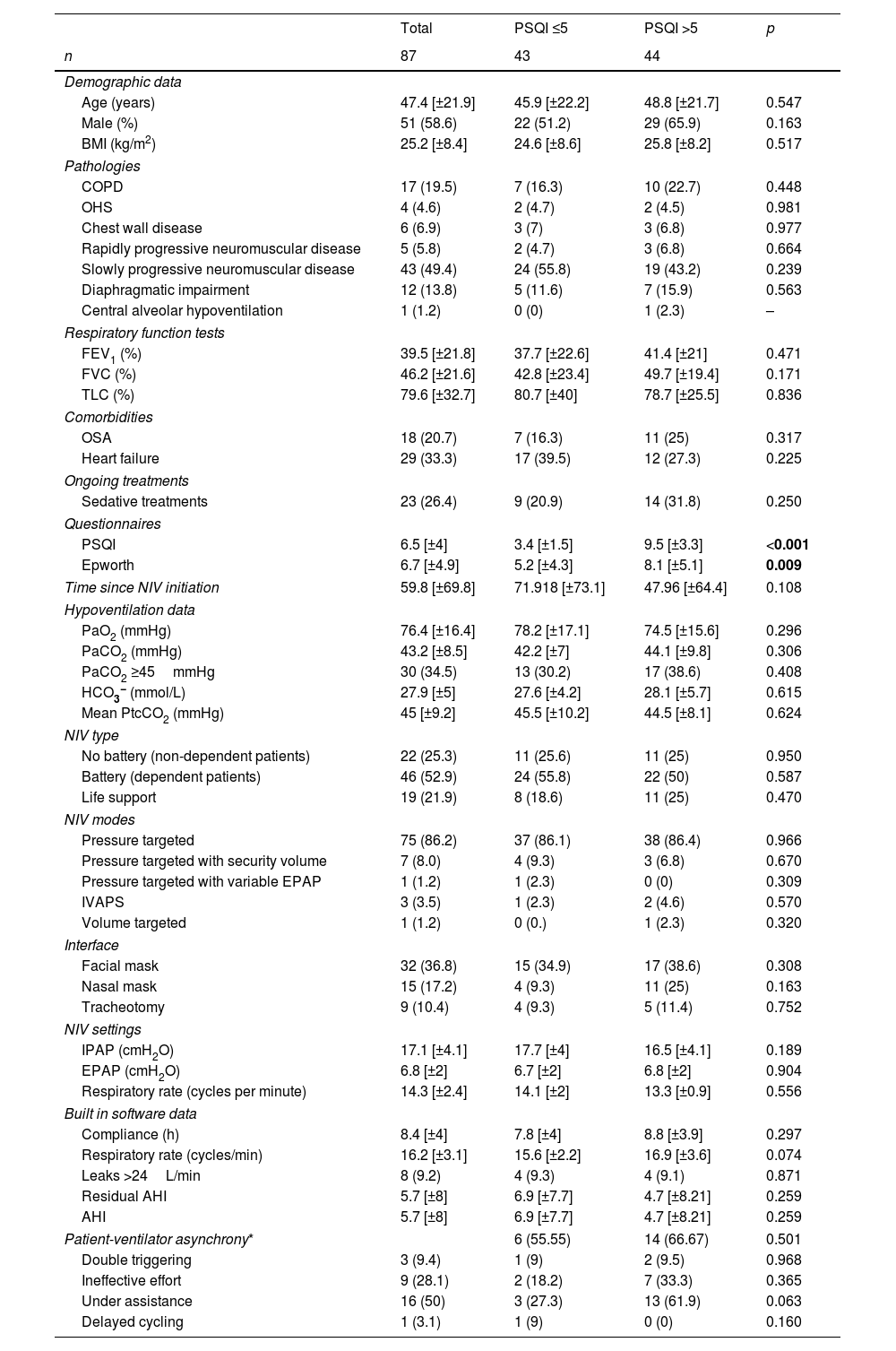
All data are presented in Table 1. In total, 87 patients were included in this study (mean age: 47.4±21.9 years; 51 [58.6%] males; mean BMI: 25.2±8.4kg/m2). The most common causes of alveolar hypoventilation were slowly progressive neuromuscular diseases (49.4%), followed by COPD (19.5%), isolated diaphragmatic impairment (13.8%), chest wall diseases (6.9%), rapidly progressive neuromuscular diseases (5.8%), and obesity hypoventilation syndrome (4.6%). Comorbidities included heart failure (33.3%), hypertension (29.9%), and sleep apnoea (20.7%). 26.4% were under sedative treatments (benzodiazepines, z-drugs, anti-psychotics). The mean PSQI score was 6.5±4, indicating that most participants had poor sleep quality (50.6%). The mean Epworth Sleepiness Scale score was 6.7±4.9, and the score was higher among patients with poor sleep quality than among those with good sleep quality (8.1±5.1 vs. 5.2±4.3, p=0.009). Arterial blood gas analysis revealed a pH of 7.4±0.04, bicarbonates level of 27.9±5mmol/L, and PaCO2 of 43.2±8.5mmHg, with residual diurnal hypoventilation seen in 30 patients (35%). The mean PtcCO2 was 45±9.2mmHg.
Patient Data.
| Total | PSQI ≤5 | PSQI >5 | p | |
|---|---|---|---|---|
| n | 87 | 43 | 44 | |
| Demographic data | ||||
| Age (years) | 47.4 [±21.9] | 45.9 [±22.2] | 48.8 [±21.7] | 0.547 |
| Male (%) | 51 (58.6) | 22 (51.2) | 29 (65.9) | 0.163 |
| BMI (kg/m2) | 25.2 [±8.4] | 24.6 [±8.6] | 25.8 [±8.2] | 0.517 |
| Pathologies | ||||
| COPD | 17 (19.5) | 7 (16.3) | 10 (22.7) | 0.448 |
| OHS | 4 (4.6) | 2 (4.7) | 2 (4.5) | 0.981 |
| Chest wall disease | 6 (6.9) | 3 (7) | 3 (6.8) | 0.977 |
| Rapidly progressive neuromuscular disease | 5 (5.8) | 2 (4.7) | 3 (6.8) | 0.664 |
| Slowly progressive neuromuscular disease | 43 (49.4) | 24 (55.8) | 19 (43.2) | 0.239 |
| Diaphragmatic impairment | 12 (13.8) | 5 (11.6) | 7 (15.9) | 0.563 |
| Central alveolar hypoventilation | 1 (1.2) | 0 (0) | 1 (2.3) | – |
| Respiratory function tests | ||||
| FEV1 (%) | 39.5 [±21.8] | 37.7 [±22.6] | 41.4 [±21] | 0.471 |
| FVC (%) | 46.2 [±21.6] | 42.8 [±23.4] | 49.7 [±19.4] | 0.171 |
| TLC (%) | 79.6 [±32.7] | 80.7 [±40] | 78.7 [±25.5] | 0.836 |
| Comorbidities | ||||
| OSA | 18 (20.7) | 7 (16.3) | 11 (25) | 0.317 |
| Heart failure | 29 (33.3) | 17 (39.5) | 12 (27.3) | 0.225 |
| Ongoing treatments | ||||
| Sedative treatments | 23 (26.4) | 9 (20.9) | 14 (31.8) | 0.250 |
| Questionnaires | ||||
| PSQI | 6.5 [±4] | 3.4 [±1.5] | 9.5 [±3.3] | <0.001 |
| Epworth | 6.7 [±4.9] | 5.2 [±4.3] | 8.1 [±5.1] | 0.009 |
| Time since NIV initiation | 59.8 [±69.8] | 71.918 [±73.1] | 47.96 [±64.4] | 0.108 |
| Hypoventilation data | ||||
| PaO2 (mmHg) | 76.4 [±16.4] | 78.2 [±17.1] | 74.5 [±15.6] | 0.296 |
| PaCO2 (mmHg) | 43.2 [±8.5] | 42.2 [±7] | 44.1 [±9.8] | 0.306 |
| PaCO2 ≥45mmHg | 30 (34.5) | 13 (30.2) | 17 (38.6) | 0.408 |
| HCO3− (mmol/L) | 27.9 [±5] | 27.6 [±4.2] | 28.1 [±5.7] | 0.615 |
| Mean PtcCO2 (mmHg) | 45 [±9.2] | 45.5 [±10.2] | 44.5 [±8.1] | 0.624 |
| NIV type | ||||
| No battery (non-dependent patients) | 22 (25.3) | 11 (25.6) | 11 (25) | 0.950 |
| Battery (dependent patients) | 46 (52.9) | 24 (55.8) | 22 (50) | 0.587 |
| Life support | 19 (21.9) | 8 (18.6) | 11 (25) | 0.470 |
| NIV modes | ||||
| Pressure targeted | 75 (86.2) | 37 (86.1) | 38 (86.4) | 0.966 |
| Pressure targeted with security volume | 7 (8.0) | 4 (9.3) | 3 (6.8) | 0.670 |
| Pressure targeted with variable EPAP | 1 (1.2) | 1 (2.3) | 0 (0) | 0.309 |
| IVAPS | 3 (3.5) | 1 (2.3) | 2 (4.6) | 0.570 |
| Volume targeted | 1 (1.2) | 0 (0.) | 1 (2.3) | 0.320 |
| Interface | ||||
| Facial mask | 32 (36.8) | 15 (34.9) | 17 (38.6) | 0.308 |
| Nasal mask | 15 (17.2) | 4 (9.3) | 11 (25) | 0.163 |
| Tracheotomy | 9 (10.4) | 4 (9.3) | 5 (11.4) | 0.752 |
| NIV settings | ||||
| IPAP (cmH2O) | 17.1 [±4.1] | 17.7 [±4] | 16.5 [±4.1] | 0.189 |
| EPAP (cmH2O) | 6.8 [±2] | 6.7 [±2] | 6.8 [±2] | 0.904 |
| Respiratory rate (cycles per minute) | 14.3 [±2.4] | 14.1 [±2] | 13.3 [±0.9] | 0.556 |
| Built in software data | ||||
| Compliance (h) | 8.4 [±4] | 7.8 [±4] | 8.8 [±3.9] | 0.297 |
| Respiratory rate (cycles/min) | 16.2 [±3.1] | 15.6 [±2.2] | 16.9 [±3.6] | 0.074 |
| Leaks >24L/min | 8 (9.2) | 4 (9.3) | 4 (9.1) | 0.871 |
| Residual AHI | 5.7 [±8] | 6.9 [±7.7] | 4.7 [±8.21] | 0.259 |
| AHI | 5.7 [±8] | 6.9 [±7.7] | 4.7 [±8.21] | 0.259 |
| Patient-ventilator asynchrony* | 6 (55.55) | 14 (66.67) | 0.501 | |
| Double triggering | 3 (9.4) | 1 (9) | 2 (9.5) | 0.968 |
| Ineffective effort | 9 (28.1) | 2 (18.2) | 7 (33.3) | 0.365 |
| Under assistance | 16 (50) | 3 (27.3) | 13 (61.9) | 0.063 |
| Delayed cycling | 1 (3.1) | 1 (9) | 0 (0) | 0.160 |
Data are expressed as mean [±standard deviation] or n (%), unless otherwise stated. AHI: Apnea-Hypopnea Index; BMI: Body Mass Index; COPD: chronic obstructive pulmonary disease; EPAP: expiratory positive airway pressure; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IPAP: inspiratory positive airway pressure; IVAPS: intelligent volume assured pressure support; NIV: non-invasive ventilation; PSQI: Pittsburgh Score Questionnaire Index; TLC: total lung capacity; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnea.
The bold values are statistically significant values: p values < 0,05.
With regard to ventilation parameters, patients were compliant (8.4±4h) and had a residual AHI of 5.7±8.20 exhibited asynchronies.
No significant difference was found between patients with good versus poor sleep quality with regards to the general characteristics (Table 1): they had the same mean age (45.9±22.2 vs. 48.8±21.7 years, p=0.547), and the same likelihood of slowly progressive neuromuscular diseases (55.8% vs. 43.2%, p=0.239), COPD (16.7% vs. 22.3%, p=0.448), and isolated diaphragmatic impairment (11.6% vs. 15.9%, p=0.563). There was no difference in terms of sedative treatments as well (20.9% vs 31.8%, p=0.250). They also had the same NIV parameters and interfaces (Table 1). Concerning NIV monitoring, they had the same compliance (7.8±4 vs. 8.8±3.9h, p=0.297), rate of important leaks (9.3% vs 9.1%, p=0.871), residual AHI (6.9±7.7 vs. 4.7±8.2, p=0.259), PaCO2 (42.2±7 vs. 44.1±9.8mmHg, p=0.306), or mean PtcCO2 (45.5±10.2 vs. 44.5±8.1mmHg, p=0.624). All types combined, there was the same percentage of asynchrony detection in both groups (55.55% vs 66.67%, p=0.501). There was no difference between the different types of asynchronies found.
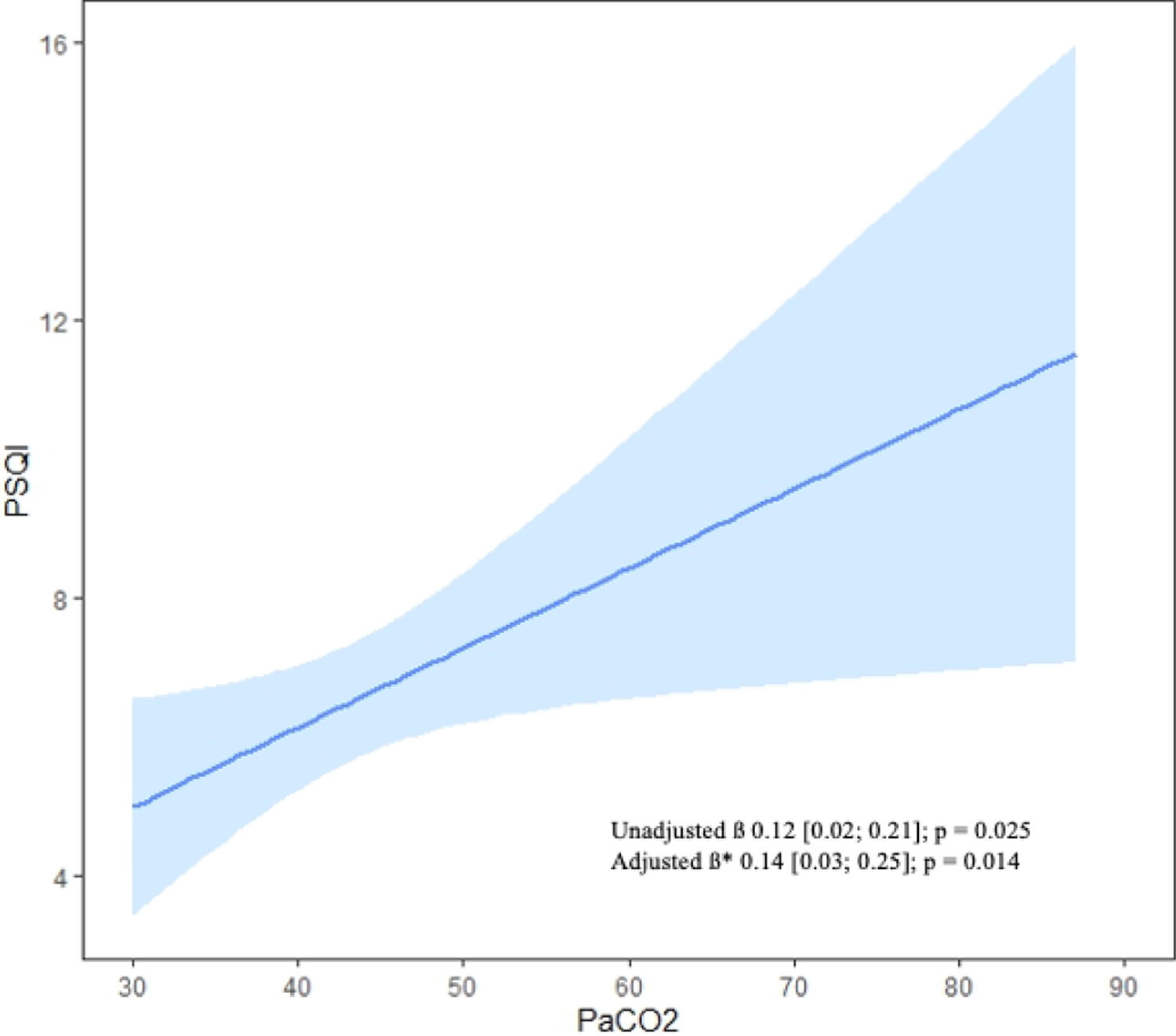
However, linear regression analysis demonstrated a significant correlation between PaCO2 and the PSQI score (β=0.12, 95% confidence interval [CI]=0.02–0.21, p=0.025), which remained significant (β=0.14, 95% CI=0.03–0.25, p=0.014; Fig. 1) after adjusting for clinically relevant variables (compliance, leaks, and AHI).
Approximately half of our study participants experienced poor sleep quality. Importantly, we identified a significant association between daytime PaCO2, as assessed by daytime arterial blood gas analysis, and PSQI scores. However, no ventilation parameter was associated with poor sleep quality. Although not statistically different, it is interesting to note that patients who sleep well have a higher residual AHI. Our results do not allow us to explain this, but we can hypothesize that patients reporting better quality sleep have more sleep cycles, and in particular more REM sleep, possibly at greater risk of an apnoeic event.10
These results are in line with those of previous studies reporting poor sleep quality in 56–66% of patients with Duchenne muscular dystrophy. Similarly, half of the participants a recent study11 had poor sleep quality, with the rate varying among COPD, obesity hypoventilation syndrome, and thoracic wall disorder groups.
We did not identify risk factors for poor sleep quality. Therefore, we cannot confirm the findings of Sutter et al.,11 who found a higher incidence of leaks in individuals with poor sleep. However, the studies enrolled different patient populations, and the threshold for evaluating leaks varied among ventilator models (between 2 and 10L/min in other studies). We used the cut off classically used to define leaks (24L/min),12 but which is higher with few patients concerned. This discrepancy highlights the need to define a relevant cut off for the definition of abnormal leakage. However, our results are in line with those of Georges et al.,7 who showed that the sleep quality of participants did not deteriorate despite inappropriate NIV treatment based on SomnoNIV guidelines.
More than half of the patients exhibited asynchronies, which is important compared to the literature,2 but it was a “self-declared” PVA detection whereas we used a medical detection strategy. There was no difference in terms of asynchrony detection between both groups, and no asynchrony was found associated with poor sleep quality. However, we report here only a summary analysis of PVA, in particular we have no polygraphy confirmation of the asynchronies reported.
We found that daytime PaCO2 was the only marker correlated with poor sleep quality, regardless of the underlying cause. The main goal of NIV is to control PaCO2 levels, and to reduce morbidity and mortality.13 Our results also emphasize that correction of hypoventilation improves sleep quality.
This work has several limitations. First of all, this is a single-centre study with a small sample and very heterogeneous population when it comes to the cause behind alveolar hypoventilation. Using the PSQI is a simple and easy to use questionnaire on a day-to-day routine for the analysis of sleep quality, recommended by French experts.14 But it lacks the information that could be gained from other questionnaires like the SRI. OSA could also interfere with the results, but because it was a small sample, we could not further analyze smaller groups of OSA patients with regards to the underlying cause for hypoventilation. No sleep difference was found according to the use of sedative treatments, despite the fact it can influence sleep quality by several means: respiratory depression, and increase of obstructive events. We did not record the presence of respiratory secretions in neuromuscular patients, which is known to worsen sleep quality,11 and can also influence the results. Finally, we have no assessment of sleep dynamics, in particular the impact of NIV initiation on sleep.
In conclusion, half of our patients who received long-term ventilation exhibited poor sleep quality. However, we did not identify any risk factors for poor sleep quality, despite comprehensive evaluation of NIV parameters. The PSQI score correlated with daytime PaCO2, highlighting the effects of PaCO2 levels on sleep quality regardless of the cause of hypoventilation.
Our results emphasize the need for a comprehensive, multidisciplinary approach to improve ventilation quality and reduce PaCO2 levels.
Conflicts of InterestThe authors have not declared a specific grant for this particular research from any funding agency in the public, commercial or not-for-profit sectors.
Pierre Schilfarth reports personal fees from SOS Oxygène, outside the submitted work.
Arnaud Maurac reports personal fees from Orkyn and personal fees from Asten, outside the submitted work.
Julie Macey reports personal fees from Isis medical, personal fees from GSK, personal fees from Chiesi, personal fees from Asten, and personal fees from SOS Oxygène, outside the submitted work.
Carole Decloedt reports personal fees from Resmed, outside the submitted work.
Maeva Zysman reports grants and personal fees from Astra Zeneca, grants and personal fees from Chiesi, grants from Avad, personal fees from GSK, personal fees from Sanofi, personal fees from CSL Behring, personal fees from Lilly, and personal fees from Menarini, outside the submitted work.
Leo Grassion reports grants and personal fees from AADAIRC, grants from FGLMR, personal fees from Vivisol, personal fees from ALMS, personal fees from Resmed, personal fees from ASV, personal fees from Asten, personal fees from SOS Oxygène, personal fees from Boerhinger Ingheleim, personal fees from Isis medical, and personal fees from Alizée santé, outside the submitted work.