Since the outbreak of the COVID-19 pandemic, numerous studies have been published regarding the treatment of pneumonia secondary to SARS-CoV-2 infection. Dexamethasone (6mg/day for 10 days maximum) administered to patients with COVID-19 pneumonia and needing ventilatory support reduced mortality at 28 days,1 while other systemic glucocorticoids have also been shown to reduce mortality in critically ill patients.2
Systemic glucocorticoids have also been studied as part of treatment for late-stage COVID-19 pneumonia, especially in the subgroup of patients with radiological and/or histological findings compatible with organizing pneumonia. For those patients, several case series3–6 and a narrative review7 have reported a satisfactory response to prolonged high-dose glucocorticoids, e.g., an initial dose of 0.75–1.5mg/kg/day of prednisone or equivalent for 2–4 weeks followed by a tapered decrease over several months.7 However, an optimal dosage regime has not yet been established for this patient subgroup, as has been done for other organizing pneumonias.8 Studies are needed to assess treatment benefits and side effects – most especially, due to its seriousness, for iatrogenic adrenal insufficiency and hypothalamic-pituitary-adrenal axis suppression. Adrenal insufficiency, which can be primary, secondary or tertiary, is an endocrine disorder characterized by adrenal hypofunction and leading to inadequate production of glucocorticoids.9 The hypothalamic-pituitary-adrenal axis controls endogenous adrenal gland cortisol production.10 Primary adrenal insufficiency results from direct adrenal gland failure caused by the destruction of or damage to the adrenal glands, secondary adrenal insufficiency occurs secondary to diseases of the pituitary gland or the hypothalamus, and tertiary adrenal insufficiency typically results from suppression of the hypothalamic-pituitary-adrenal axis, caused by the administration of supraphysiological doses of exogenous glucocorticoids over a sustained period of time.9
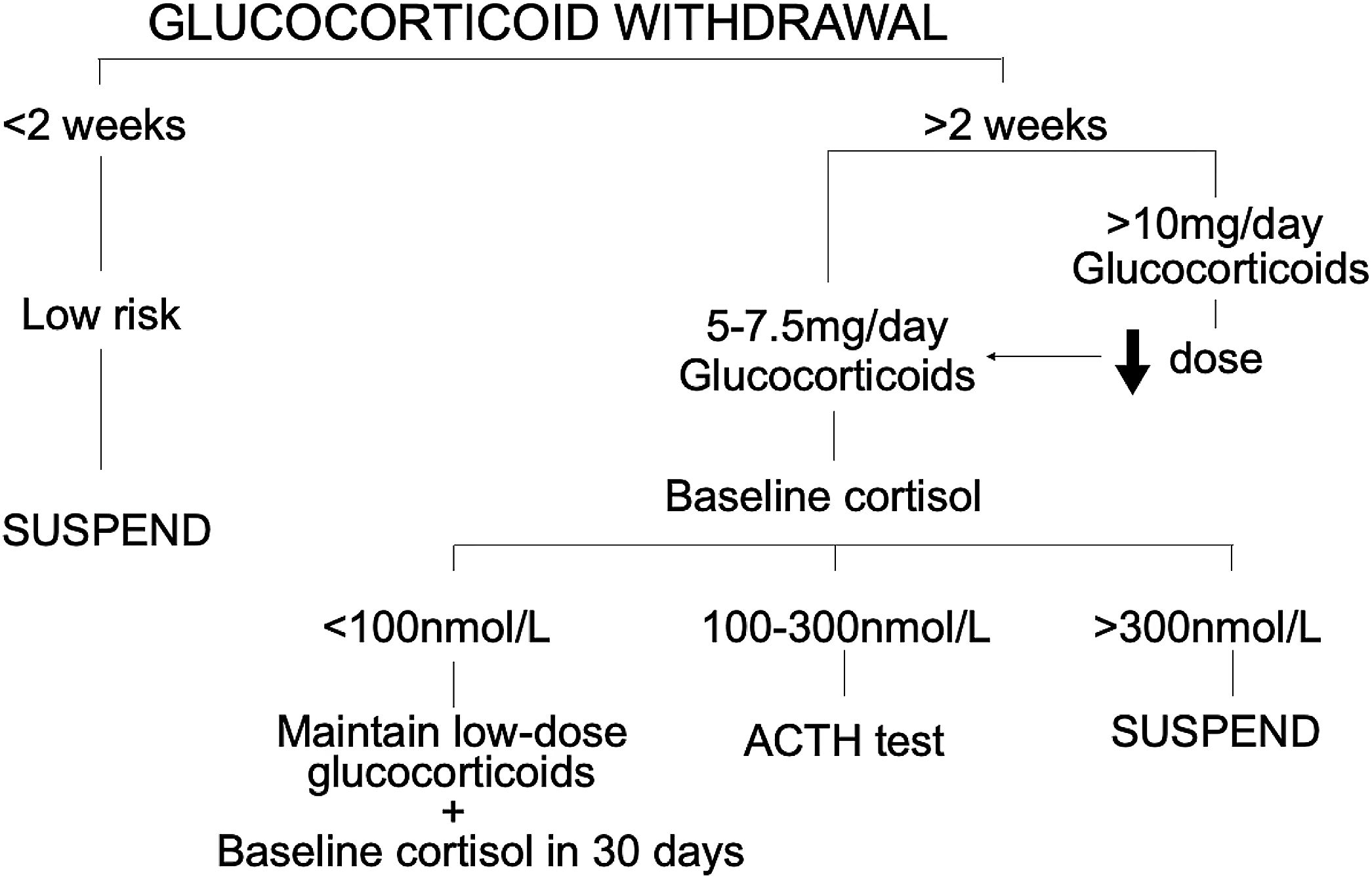
We describe a single-centre retrospective study of patients treated with systemic glucocorticoids over a prolonged period of hospitalization for post COVID-19 organizing pneumonia (confirmed by high-resolution computed tomography). All patients who had post-COVID-19 organized pneumonia were discharged with a follow-up visit at the Respiratory Day Hospital. The study included all patients seen during 2021. A total of 43 patients underwent clinical and radiological follow-up to monitor glucocorticoid tapering. Prior to complete withdrawal, and following our hospital glucocorticoid withdrawal protocol (Fig. 1), baseline cortisol levels were measured. Administered, if deemed necessary, was a synthetic ACTH stimulation test (tetracosactide) to detect abnormal cortisol or 17-hydroxyprogesterone secretions in cortisol. Measurements were made at 8–9 in the morning (cortisol-1-ACTH) and again 60min later (cortisol-2-ACTH) in order to determine whether the adrenal response to stress was adequate.
Of the 43 patients in our series, 64.2% were men with a mean age of 67 years and a mean body mass index (BMI) of 27.6kg/m2. The most frequent comorbidity was arterial hypertension (56.1%), followed at a distance by chronic obstructive pulmonary disease (16.6%) and diabetes mellitus (14.2%). Hospitalization lasted a mean of 23.5 (±18.4) days and the mean cumulative dose of oral glucocorticoids was 1558.8 (±964.3)mg administered for a mean of 83.2 (±53) days. Data on the cumulative dose of glucocorticoids and the number of days glucocorticoids were administered were obtained by retrospectively collecting data from patient clinical histories. Mean baseline cortisol levels were 286.9nmol/L (<100nmol/L in 14.7% of the patients). The ACTH stimulation test was administered to 54.5% of the patients, whose levels were, at baseline, 265.8nmol/L (minimum-maximum 58–393nmol/L), and after 60min, 547.2nmol/L (minimum–maximum 204–843nmol/L). Adrenal insufficiency prevalence was 14.7% and hypothalamic-pituitary-adrenal axis suppression prevalence was 47%. The patients did not present symptoms of adrenal insufficiency, and no directed anamnesis was performed. Indeed, because such symptoms are nonspecific, they are often overlooked or misdiagnosed.9 By the end of our study, persistent symptoms or continued adrenal insufficiency meant that corticosteroid therapy could not be withdrawn in 23.5% of the patients.
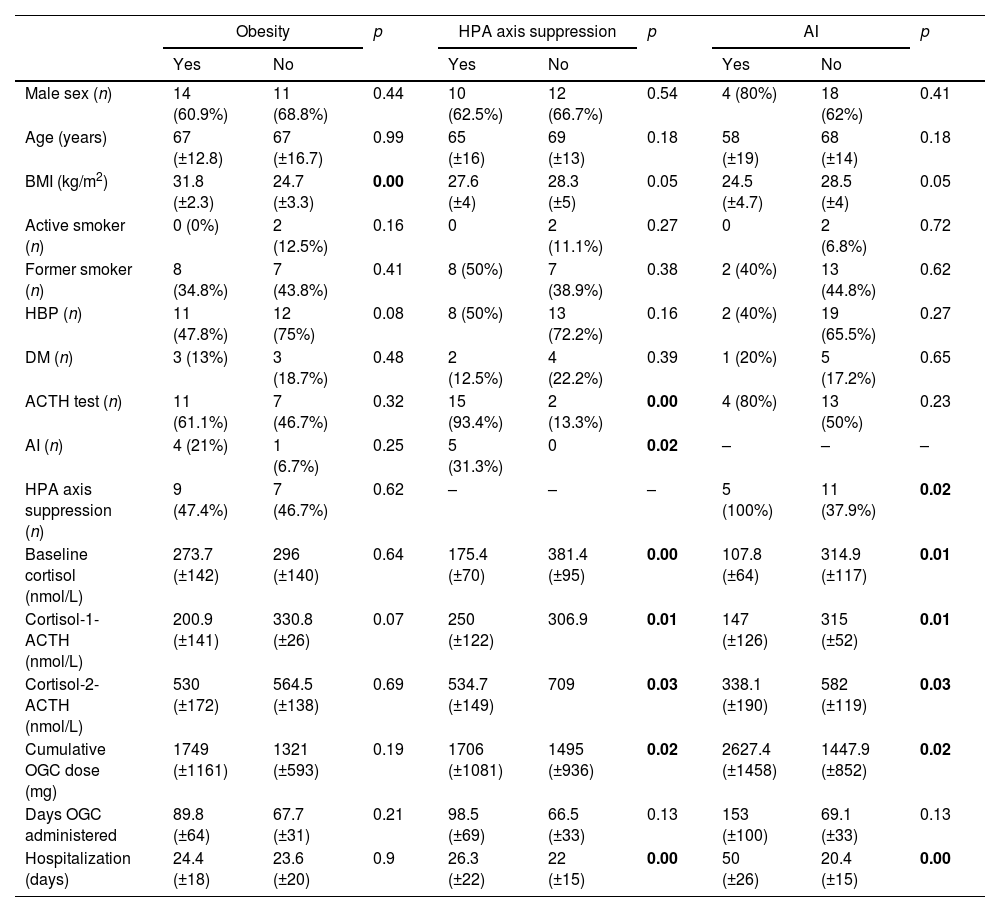
Pearson correlation coefficient (r) was calculated and significant positive correlations were found for the cumulative dose of oral glucocorticoids with the number of hospitalization days (r=0.361, p=0.03) and BMI with cortisol-1-ACTH (r=0.83, p=0.01), while significant inverse correlations were found for baseline cortisol levels with the number of glucocorticoid treatment days (r=−0.39, p=0.02), and likewise, for cortisol-1-ACTH and cortisol-2-ACTH with the number of glucocorticoid treatment days (r=−0.64, p=0.01; r=−0.60, p=0.02). In other words, the more hospitalization days, the greater the cumulative dose of oral glucocorticoids; and the greater the number of glucocorticoid administration days, the lower the cortisol levels both before and after administration of ACTH. Note that higher BMI scores were associated with higher cortisol-1-ACTH levels, but not with baseline or cortisol-2-ACTH levels. Table 1 summarizes patient variables in relation to obesity, hypothalamic-pituitary-adrenal axis suppression, and adrenal insufficiency.
Patient variables as a function of obesity, hypothalamic-pituitary-adrenal axis suppression and adrenal insufficiency.
| Obesity | p | HPA axis suppression | p | AI | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||
| Male sex (n) | 14 (60.9%) | 11 (68.8%) | 0.44 | 10 (62.5%) | 12 (66.7%) | 0.54 | 4 (80%) | 18 (62%) | 0.41 |
| Age (years) | 67 (±12.8) | 67 (±16.7) | 0.99 | 65 (±16) | 69 (±13) | 0.18 | 58 (±19) | 68 (±14) | 0.18 |
| BMI (kg/m2) | 31.8 (±2.3) | 24.7 (±3.3) | 0.00 | 27.6 (±4) | 28.3 (±5) | 0.05 | 24.5 (±4.7) | 28.5 (±4) | 0.05 |
| Active smoker (n) | 0 (0%) | 2 (12.5%) | 0.16 | 0 | 2 (11.1%) | 0.27 | 0 | 2 (6.8%) | 0.72 |
| Former smoker (n) | 8 (34.8%) | 7 (43.8%) | 0.41 | 8 (50%) | 7 (38.9%) | 0.38 | 2 (40%) | 13 (44.8%) | 0.62 |
| HBP (n) | 11 (47.8%) | 12 (75%) | 0.08 | 8 (50%) | 13 (72.2%) | 0.16 | 2 (40%) | 19 (65.5%) | 0.27 |
| DM (n) | 3 (13%) | 3 (18.7%) | 0.48 | 2 (12.5%) | 4 (22.2%) | 0.39 | 1 (20%) | 5 (17.2%) | 0.65 |
| ACTH test (n) | 11 (61.1%) | 7 (46.7%) | 0.32 | 15 (93.4%) | 2 (13.3%) | 0.00 | 4 (80%) | 13 (50%) | 0.23 |
| AI (n) | 4 (21%) | 1 (6.7%) | 0.25 | 5 (31.3%) | 0 | 0.02 | – | – | – |
| HPA axis suppression (n) | 9 (47.4%) | 7 (46.7%) | 0.62 | – | – | – | 5 (100%) | 11 (37.9%) | 0.02 |
| Baseline cortisol (nmol/L) | 273.7 (±142) | 296 (±140) | 0.64 | 175.4 (±70) | 381.4 (±95) | 0.00 | 107.8 (±64) | 314.9 (±117) | 0.01 |
| Cortisol-1-ACTH (nmol/L) | 200.9 (±141) | 330.8 (±26) | 0.07 | 250 (±122) | 306.9 | 0.01 | 147 (±126) | 315 (±52) | 0.01 |
| Cortisol-2-ACTH (nmol/L) | 530 (±172) | 564.5 (±138) | 0.69 | 534.7 (±149) | 709 | 0.03 | 338.1 (±190) | 582 (±119) | 0.03 |
| Cumulative OGC dose (mg) | 1749 (±1161) | 1321 (±593) | 0.19 | 1706 (±1081) | 1495 (±936) | 0.02 | 2627.4 (±1458) | 1447.9 (±852) | 0.02 |
| Days OGC administered | 89.8 (±64) | 67.7 (±31) | 0.21 | 98.5 (±69) | 66.5 (±33) | 0.13 | 153 (±100) | 69.1 (±33) | 0.13 |
| Hospitalization (days) | 24.4 (±18) | 23.6 (±20) | 0.9 | 26.3 (±22) | 22 (±15) | 0.00 | 50 (±26) | 20.4 (±15) | 0.00 |
HPA, hypothalamic-pituitary-adrenal; BMI, body mass index; HBP, high blood pressure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; ACTH, adrenocorticotropic hormone; AI, adrenal insufficiency; OCG, oral glucocorticoids. Bold values indicated they are statistically significant.
To our knowledge, no studies have been published on the prevalence of iatrogenic adrenal insufficiency in patients with post COVID-19 organizing pneumonia treated with oral glucocorticoids. In our series, prevalence was 14.7%, indicating that those patients might have experienced an adrenal crisis if glucocorticoid treatment had been completely withdrawn before baseline cortisol levels were measured.
One of the limitations of this study is the small sample drawn from a single centre. Furthermore, since the glucocorticoids were prescribed at the discretion of the individual physicians caring for each patient during hospitalization, the cumulative dose varied greatly between patients.
The interest of this letter lies in the limited literature available on the effects on the adrenal gland of prolonged high-dose systemic glucocorticoid treatment in patients with post COVID-19 organizing pneumonia. Given the high prevalence of adrenal insufficiency observed in our series, we recommend that studies be conducted of side effects, as this would enable the development of a risk-benefit profile for glucocorticoid treatment and a protocol for baseline cortisol measurement prior to complete treatment withdrawal.
FundingThe translation of this article was financed by Instituto de Salud Carlos III, PI17/01950, and co-funded by European Union (FEDER).