In patients with obstructive sleep apnea (OSA), novel metrics such as hypoxic burden (HB) and sleep apnea-specific pulse-rate response (ΔHR) may better correlate with cardiovascular diseases (CVD) than the apnea–hypopnea index (AHI). This manuscript aims to assess the correlation between ΔHR and HB with subclinical atherosclerosis in patients with OSA, testing the hypothesis that elevated ΔHR and HB are associated with subclinical atherosclerosis development.
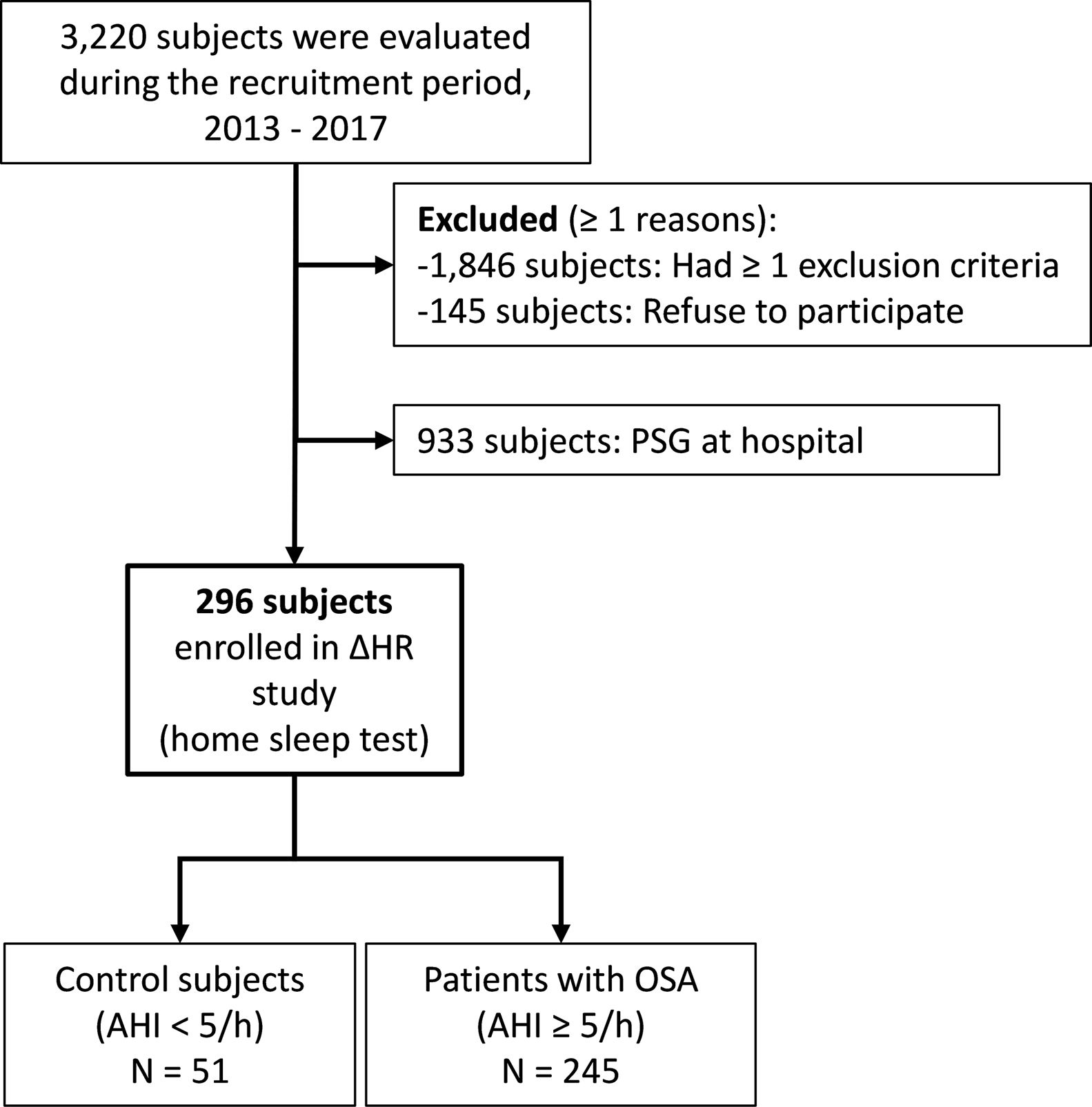
MethodsIn a prospective study, individuals aged 20–65 years with suspected OSA without known comorbidities were consecutively recruited and defined as OSA (AHI≥5events/h) or healthy controls. Using bilateral carotid ultrasonography, common carotid intima-media thickness (CIMT) was assessed and the identification of at least one atheromatous plaque defined the presence of subclinical atherosclerosis. ΔHR, and HB were derived from pulse-oximetry.
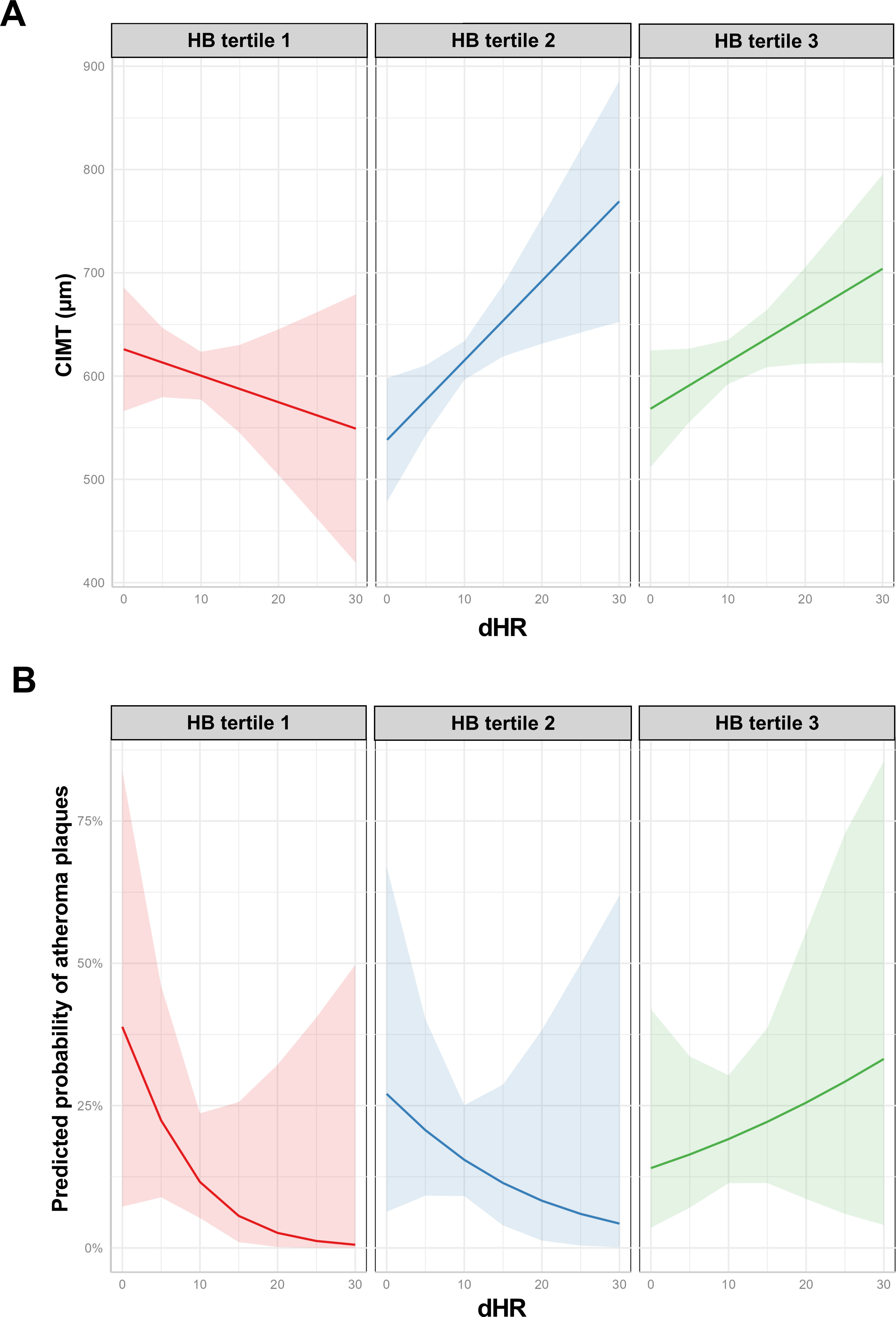
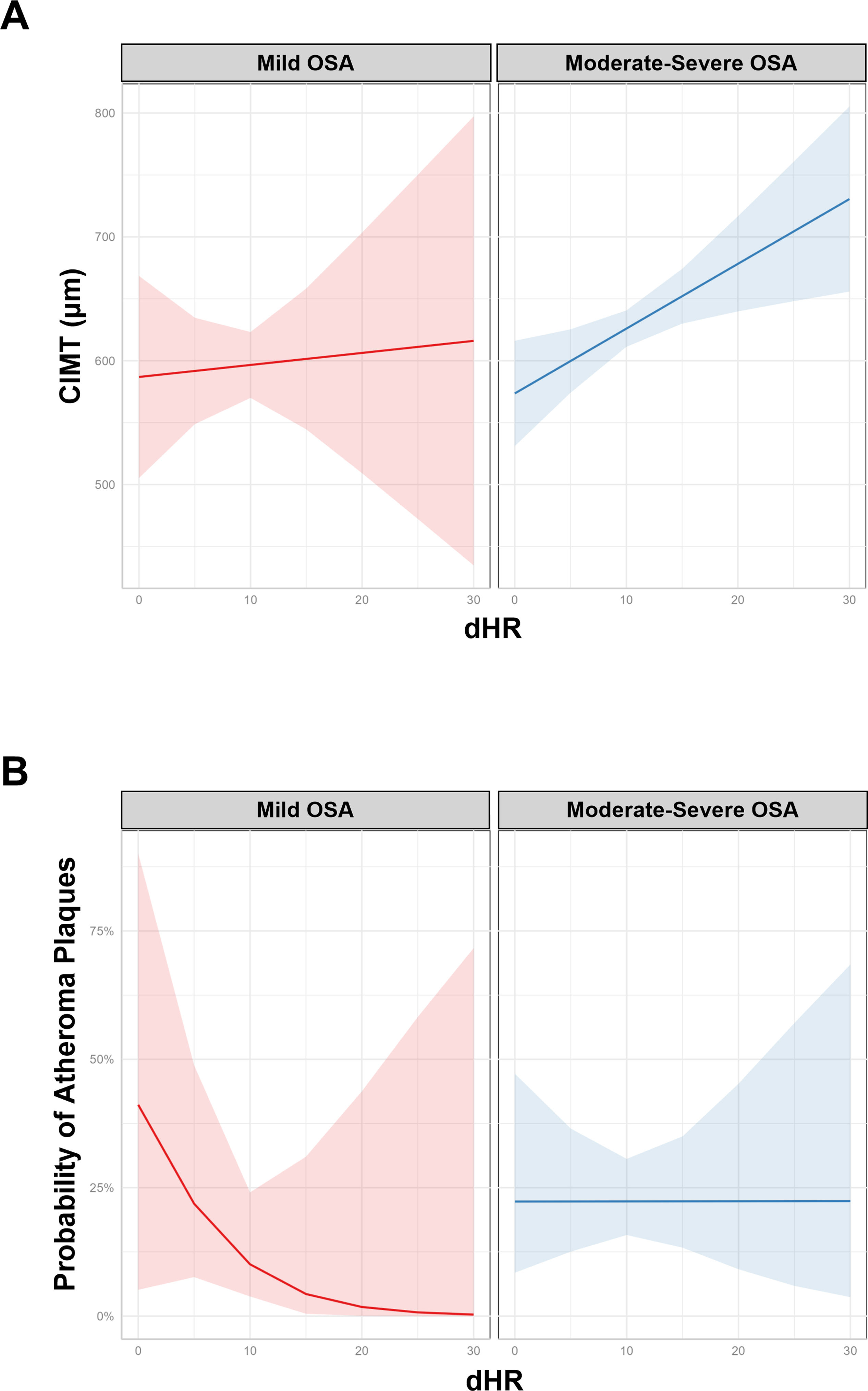
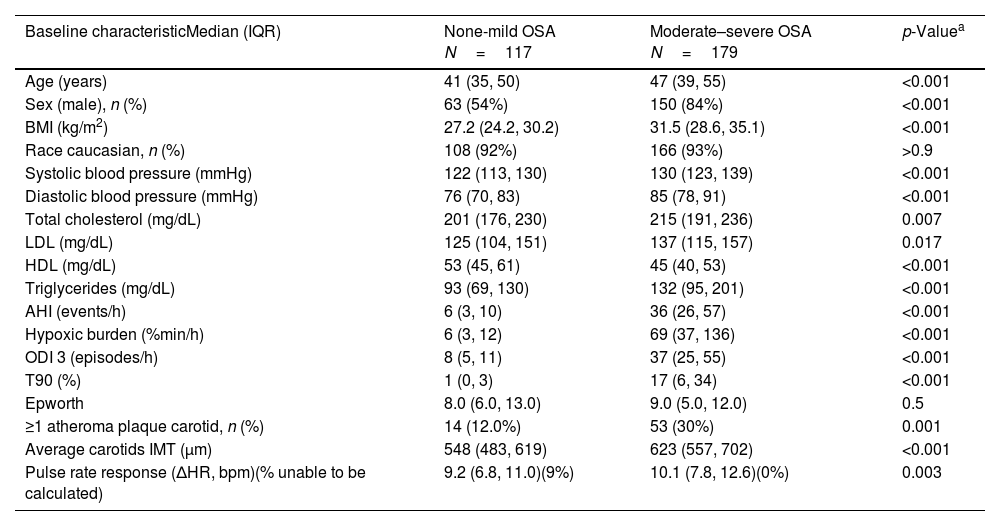
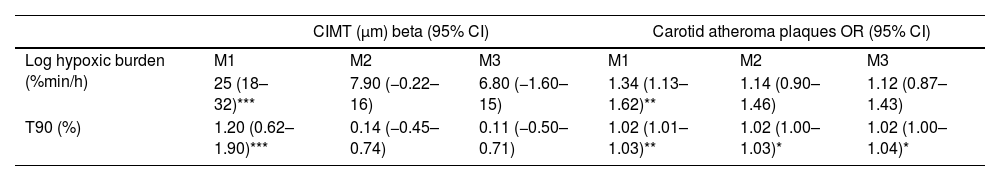
ResultsWe studied 296 patients of age 45±10 years old, of whom 28% were women, and with a BMI of 30.3±5.3kg/m2. Overall, 245 had OSA and 51 were healthy controls. After controlling for confounding variables higher ΔHR but not HB, was associated with higher CIMT (p=0.006) and higher time spent with oxygen saturation below 90% (T90) was associated with an increase in carotid atheroma plaques (p=0.032). When stratifying OSA based on HB tertiles, we observed that within tertile 2 of HB, an increase in ΔHR was associated with larger CIMT (p=0.017).
ConclusionA higher ΔHR is associated with an increase in CIMT among adult patients with OSA. This study suggests that ΔHR could be a biomarker of risk for CVD in patients with OSA.