Contrast-enhanced ultrasound is of great utility in evaluating lung lesions that are in peripheral locations or contained within a consolidation1, since it helps identify areas of necrosis and thus improves diagnostic accuracy when obtaining samples2. Similarly, contrast-enhanced studies help reach a differential diagnosis, as they highlight specific characteristics of certain diseases, such as pulmonary infarction, atelectasis, aggressive cancers, and obstructive atelectasis due to central lesions2.
The time to enhancement of the parenchyma or lung lesion varies, depending on whether the vascular component is supplied from the pulmonary arteries (<6 s) or from the bronchial arteries (>6 s). Similarly, the pattern and extent of enhancement as well as the washout time (greater than 60 s) in a consolidated focus or lung lesion helps differentiate between lung collapse, infectious process/abscess, infarction, and tumor. In general, tumors receive arterial vascular supply from the bronchial arteries, as the pulmonary arteries are incapable of neoangiogenesis. The delay in uptake by malignant lesions is explained by intrinsic vasoconstrictions, given the intrinsic hypoxic status of the neoproliferative lesion. Benign lesions, in contrast, receive blood from both the pulmonary and bronchial arteries, and therefore show early enhancement3.
Thus, in the case of passive atelectasis, B-mode shows a homogeneous consolidation containing hyperechoic air bronchogram and early arterial enhancement that persists throughout the examination and may remain for longer than 5 min4. The findings for areas of pulmonary infarction will be similar to those of atelectasis in B-mode and we can identify hypoechoic nodules within the area of collapse. After the administration of contrast agent, we will see an absence of enhancement in the infarcted areas4. Pulmonary abscesses show delayed enhancement (>6 s) with hypoechoic and hypodense central areas corresponding to necrosis, which may appear in preexisting tumors4.
Pneumonia and metastatic lesions and malignant lesions are a diagnostic challenge, especially in cases where both entities coexist. Pneumonias in general show early (<6 s) homogeneous arterial enhancement. However, in some cases both pathologies show delayed enhancement (>6 s). They differ in that in pneumonia, homogeneous enhancement is maintained in late phases with late washout (>60 s), while metastatic lesions will show faster washout (<60 s) of the lesion than the surrounding parenchyma4.
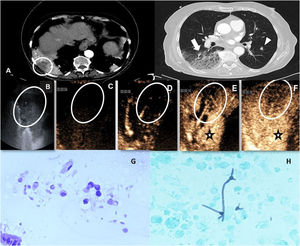
We report the case of a 79-year-old woman with a history of papillary thyroid carcinoma who attended the emergency room for dyspnea that had worsened progressively until it appeared with minimal exertion. Chest X-ray revealed multiple foci of consolidation. A computed tomography (CT) scan was performed that ruled out pulmonary thromboembolism but revealed heterogeneous consolidations in both lower lobes along with areas suggestive of infectious bronchiolitis and some solid nodules (Fig. 1A). A positive test was obtained for tumor markers (CA 19-9 and CYFRA 21-1) and, in view of a suspected malignant origin, a positron emission tomography (PET/CT) with fluorodeoxyglucose was performed, which showed probable pancreatic cancer and possible metastatic consolidations in the lungs. Since the largest consolidation, located in the lower right lobe, was in extensive contact with the peripheral pleura, we decided to perform ultrasound-guided biopsy. We administered 2.4 mL of ultrasound contrast agent (SonoVue, Rovi, Pozuelo de Alarcón, Madrid, Spain) and two distinct areas were observed, differentiated by their uptake pattern: a peripheral area with delayed enhancement (>6 s post-injection) and early washout (disappearance of contrast uptake within a few seconds of uptake), while the remaining consolidation showed early homogeneous enhancement (<6 s) and delayed washout (>1 min) (Fig. 1B–F). In view of these findings, we decided to biopsy the first of these areas with a 22 G fine needle; it was reported as alveolar metastasis of pancreatic adenocarcinoma (Fig. 1G). Because the rest of the consolidation showed suggestive characteristics of a pneumonia process, we decided to perform fiberoptic bronchoscopy; samples obtained showed a fungal infection (Fig. 1H).
(A) CT after intravenous iodinated contrast agent. Axial slices are shown in the mediastinum window (left) and pulmonary parenchyma window (right) where heterogeneous consolidation is observed in the right lower lobe with hypodense areas (circle) and areas of ground glass and tree-in-bud pattern (arrow). Some bilateral solid pulmonary nodules (arrowhead) probably associated with metastases are also observed. (B) B-mode ultrasound performed in the same patient showing heterogeneous consolidation without air bronchogram and anechoic subpleural area (oval). (C–F) Ultrasound image after injection of contrast agent showing homogeneous but delayed enhancement (star) of the consolidated pulmonary parenchyma (pneumonia pattern). A subpleural triangular area is observed that shows more delayed enhancement than the surrounding consolidated parenchyma (oval). Moreover, washout of this area is rapid, suggesting malignancy. An ultrasound-guided biopsy of the suspicious area was subsequently performed with histological results confirming pancreatic adenocarcinoma metastases. Later, a fiberoptic bronchoscopy confirmed fungal superinfection in the surrounding parenchyma. (G) Large atypical and vacuolated epithelial cell groups associated with metastatic pancreatic adenocarcinoma. (H) Microbiological analysis of the bronchoalveolar lavage sample from the consolidation area showing branched structures corresponding to hyphae.
In our patient we were able to differentiate between pneumonia and metastasis, as she presented pre-existing pulmonary consolidation containing a hypoechoic area. On contrast-enhanced ultrasound, the consolidation showed late homogeneous uptake with delayed washout, with the exception of the central and peripheral areas that showed late enhancement but early washout, suggestive of malignancy. This enhancement pattern also helped guide the percutaneous biopsy to the most suspicious target area to improve the diagnostic yield of the samples collected.
Ultrasound-guided biopsy is an alternative to CT-guided biopsy for peripheral or pleural lung lesions1,3 and achieves a similar diagnostic effectiveness and yield as CT5. Additionally, ultrasound-guided percutaneous procedures offer certain advantages, such as real-time monitoring of the procedure, absence of radiation, lower costs and duration of the procedure, and the complication rates are similar to or lower than with CT-guided biopsy1,2. In many cases, contrast-enhanced chest ultrasound helps clarify the nature of the lesion under study and, if necessary, guides the biopsy needle towards areas of interest, avoiding necrotic foci1,2,6 and targeting areas with a greater suspicion of malignancy, as in the case presented.
Please cite this article as: Isus Olivé G, Páez Carpio A, Martínez D, Vollmer I. Papel de la ecografía con contraste en la diferenciación entre una neumonía y una neoplasia en el seno de una consolidación pulmonar. Arch Bronconeumol. 2021;57:605–607.