Inhaled therapy is the mainstay of pharmacological treatment in patients with chronic obstructive pulmonary disease (COPD)1. The Spanish COPD guidelines (GesEPOC)2 recommends tailoring pharmacological treatment to the patient’s characteristics, level of symptoms, and risk of exacerbations. The Global Strategy for the Management of COPD (GOLD)3 also focuses on a personalized approach, and since its 2019 update has proposed the use of algorithms for the choice of initial treatment and maintenance4. These novel proposals recommend a dynamic assessment and adjustment of treatment throughout follow-up with the application of intensification or reduction strategies (escalation/de-escalation), especially in patients who, despite a correct inhalation technique and adherence to the prescribed treatment, fail to achieve adequate disease control.
While these therapeutic strategies are primarily aimed at improving the adequacy of pharmacological treatment over the course of the disease, they can require certain changes, not only in the drugs administered, but also in the inhalation devices that deliver them. According to the consensus document on inhaled therapy prepared by the European Respiratory Society and the International Society for Aerosols in Medicine, when a patient is familiar with a device and is performing the inhalation technique correctly, that device should not be changed, unless the patient agrees and is taught how to use the new system5. This recommendation is based on the fact that changing inhalation systems may lead to errors in use, especially if different inhalation techniques are required, resulting in a lack of therapeutic compliance6–8.
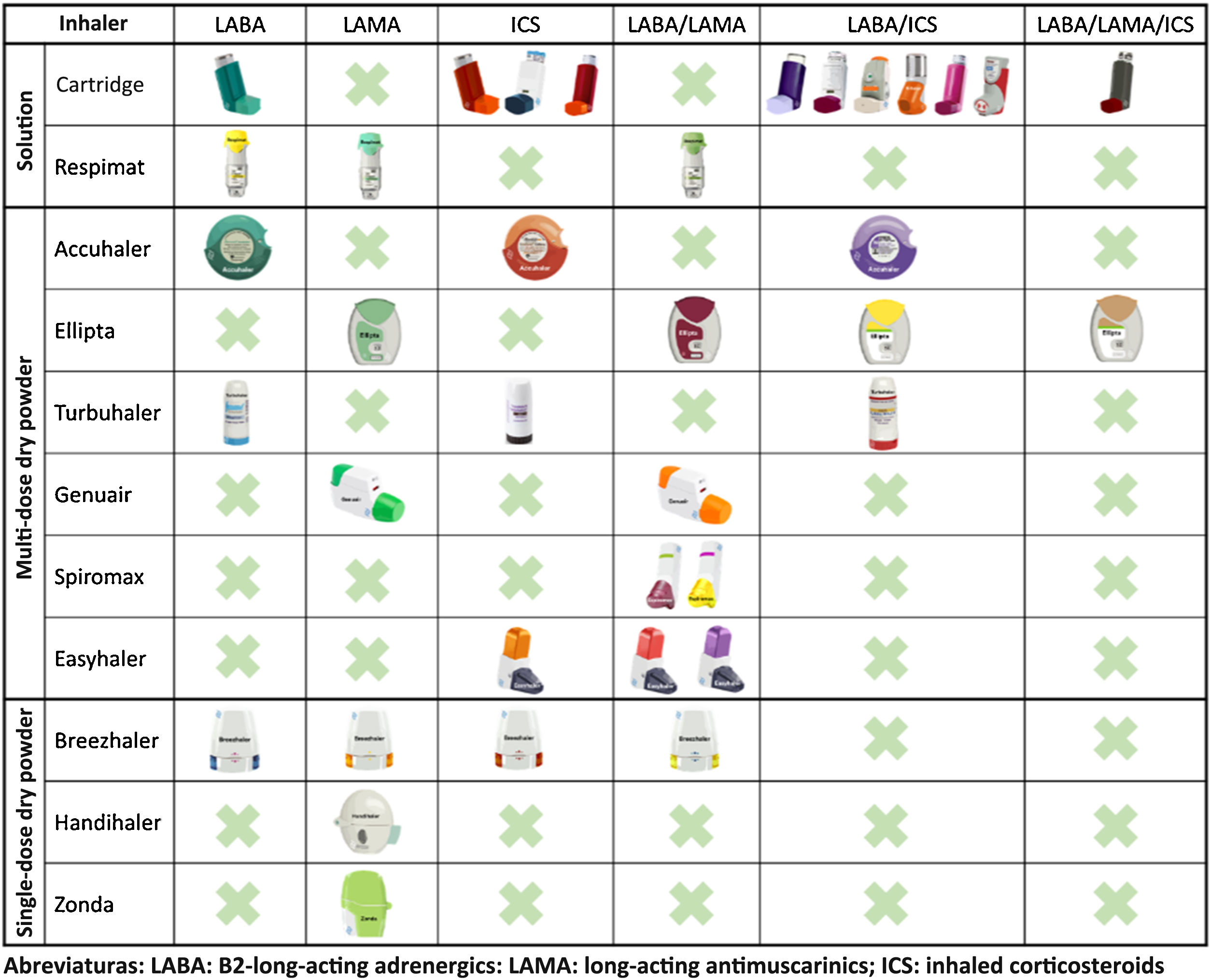
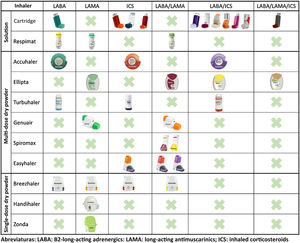
In recent years, remarkable advances in drug development have led to the emergence of new molecules and inhalation devices for the treatment of COPD. However, if we look at the main inhalation systems on the market, we can see that no single device can yet be used with all the drug groups needed for the treatment of this disease (long-acting bronchodilators and inhaled corticosteroids in monotherapy and in double and triple combinations) (Fig. 1). This means that in order to apply escalation and de-escalation strategies during follow-up, it is sometimes impossible to avoid switching inhalation devices or using different combinations.
When selecting an inhalation device in this scenario, it would be logical to bear in mind not only the current treatment, but also possible changes in the future. To this end, choosing inhalation devices that have a similar mechanism of action should be considered as yet another variable in the selection of pharmacological treatment. When selecting inhalers for solutions, the combination of pressurized cartridges with Respimat® covers all possible combinations. Among the multi-dose dry powder devices, the Accuhaler® and Ellipta® combination would also cover all therapeutic options using devices that have similar mechanisms of action. For single-dose dry powder devices, no combination that covers all options will be available until the development of the Breezhaler® with the LABA/ICS combination is completed9 or triple therapy becomes available.
Each inhalation device has its advantages and disadvantages, so the choice of one or the other should be individualized and determined primarily by the patient’s characteristics, the medication they require, the ease with which they use the device, and their own preferences10,11. Moreover, based on the above, we believe that escalating or de-escalating pharmacological treatment in COPD should be a further factor to consider when choosing an inhalation device. In the future, we must prioritize therapeutic options that allow us to switch medications during follow-up, while changing the device as seldom as possible. This will help achieve greater clinical effectiveness over the course of the disease.
Please cite this article as: Alonso-Pérez T, García-Castillo E, López-Campos JL. Escalando y desescalando el tratamiento en la enfermedad pulmonar obstructiva crónica. ¿El inhalador importa? Arch Bronconeumol. 2021;57:604–605.