During the Covid-19 pandemic, a lack of ventilatory equipment in intensive care units (ICU), or patient comorbidities meant that some patients received non invasive, continuous positive airway pressure (CPAP), with the highest fraction of inspired oxygen (FiO2) as a ceiling treatment.1–3 In Le Havre hospital (France), between September and December 2020, around 30 patients were treated with bilevel home devices in CPAP mode using vented oronasal masks. In contrast with bench studies that reported high FiO2 with oxygen flow rates<30L/min in optimal experimental conditions (i.e., low minute ventilation, good pulmonary compliance and no leakage),4,5 many patients required O2 flow rates>70L/min to maintain oxygen saturation (SpO2)≥90%. We hypothesised that due to the high respiratory demand of patients with severe Covid-19, a high O2 flow rate would be required to reach adequate pressure levels with CPAP, thereby substantially increasing the oxygen flow rate needed to achieve a high FiO2. We carried out a bench study to measure the oxygen flow rates needed to reach high FiO2 levels, using a pulmonary model that reproduced the characteristics of patients with severe Covid-19.
First, we extracted clinical data from 10 consecutive patients who were included in an ongoing clinical trial (EURO-CPAP CT2220141 approved by our institutional review board) from the CPAP built-in software. This case series is presented for descriptive purposes to illustrate our hypothesis. Then, we performed several bench experiments. We used a CPAP device (AirSense 10 AutoSet, ResMed, San Diego, CA, USA) set at 10 cmH2O, connected to an artificial lung (ASL 5000, Ingmar Medical, Pittsburgh, PA, USA). A low-resistance antibacterial filter (Clear-Guard, Intersurgical, Wokingham, UK), an oxygen inlet connector, a 15mm single limb circuit, a Whisper Swivel II Exhalation port (Philips Respironics, Murrysville, PA, USA) that simulated intentional leaks, and the artificial lung were placed in series and connected to the CPAP device. A standard 4-mm diameter leak port was placed between the exhalation port and the artificial lung to mimic unintentional leakage. Oxygen was delivered into the system using two O2 flow meters (EASY MED-O2, Air Liquide Healthcare, Paris, France) that could both deliver up to 50L/min. Three different mechanical lung conditions were simulated by modulating the resistance (R) and the compliance (C) of the artificial lung, corresponding to the following experimental models:
- A.
Normal: R=5cmH2O/ls and C=60ml/cmH2O.
- B.
Restrictive: R=5 cmH2O/ls and C=30ml/cmH2O.
- C.
Normal with by-pass leak using a T-connector as recommended to reduce the risk of droplet aerosolization.6
Each model was run at breathing frequencies of 22, 30 and 35 cycles per minute (cpm), associated with inspiratory airway pressure drops of 2.5–6cmH2O at 100ms (P0.1) to reach tidal volumes of around 600ml. The values of the inspiratory effort settings chosen for the simulation in the ASL5000 were based on published clinical values.7–11 Each condition was run with and without unintentional leakage. FiO2 was measured with 5, 15, 30, 50, 60 and 80L/min of O2, and the experiment was stopped when 100% FiO2 was reached. The sensor used was a paramagnetic oxygen transducer located in the ASL 5000 piston chamber which allowed a fast measurement with a response time below 350ms. Ten respiratory cycles were analysed after the FiO2 had reached an asymptote for each model at each O2 flow rate. Normality and homogeneity were assessed using Shapiro-Wilk and Levene tests, respectively. Welch's ANOVA was used to compare respiratory rates at each O2 flow rate. A Games-Howell post-hoc procedure was applied for multiple comparisons, with 22cpm as the reference condition. Significance was set at 0.05. Statistical analysis was performed using IBM SPSS Statistics V25.
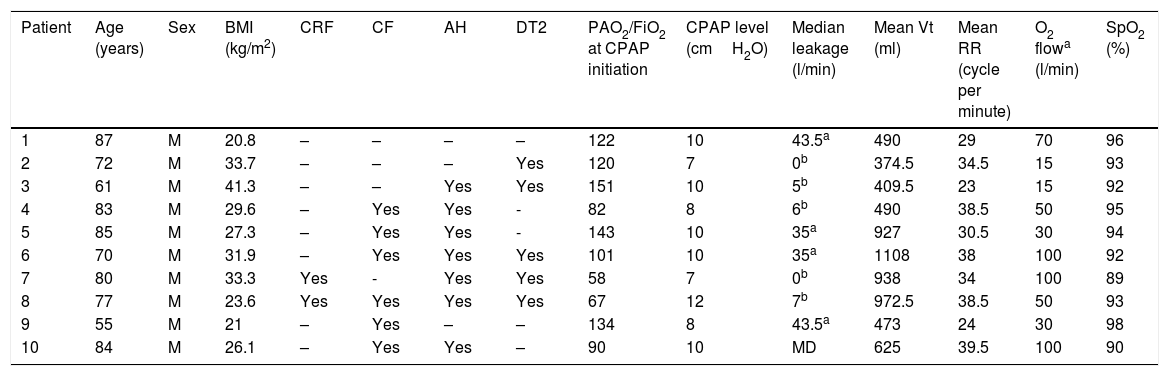
Data from 10 consecutive patients with Covid-19 treated with CPAP and O2 are presented in Table 1. Six patients needed O2 flow rates≥50L/min to maintain SpO2>90%. Minimum O2 flow rate was 15L/min. All patients had severe infection (i.e., PaO2/FiO2 ratio≤150at CPAP initiation).
Descriptive data from 10 consecutive patients treated with CPAP and O2. Leakage, Tidal Volume (Vt) and Respiratory Rate (RR) were collected from built-in CPAP software during the first 48h of CPAP treatment.
| Patient | Age (years) | Sex | BMI (kg/m2) | CRF | CF | AH | DT2 | PAO2/FiO2 at CPAP initiation | CPAP level (cmH2O) | Median leakage (l/min) | Mean Vt (ml) | Mean RR (cycle per minute) | O2 flowa (l/min) | SpO2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 87 | M | 20.8 | – | – | – | – | 122 | 10 | 43.5a | 490 | 29 | 70 | 96 |
| 2 | 72 | M | 33.7 | – | – | – | Yes | 120 | 7 | 0b | 374.5 | 34.5 | 15 | 93 |
| 3 | 61 | M | 41.3 | – | – | Yes | Yes | 151 | 10 | 5b | 409.5 | 23 | 15 | 92 |
| 4 | 83 | M | 29.6 | – | Yes | Yes | - | 82 | 8 | 6b | 490 | 38.5 | 50 | 95 |
| 5 | 85 | M | 27.3 | – | Yes | Yes | - | 143 | 10 | 35a | 927 | 30.5 | 30 | 94 |
| 6 | 70 | M | 31.9 | – | Yes | Yes | Yes | 101 | 10 | 35a | 1108 | 38 | 100 | 92 |
| 7 | 80 | M | 33.3 | Yes | - | Yes | Yes | 58 | 7 | 0b | 938 | 34 | 100 | 89 |
| 8 | 77 | M | 23.6 | Yes | Yes | Yes | Yes | 67 | 12 | 7b | 972.5 | 38.5 | 50 | 93 |
| 9 | 55 | M | 21 | – | Yes | – | – | 134 | 8 | 43.5a | 473 | 24 | 30 | 98 |
| 10 | 84 | M | 26.1 | – | Yes | Yes | – | 90 | 10 | MD | 625 | 39.5 | 100 | 90 |
Body mass index: BMI; chronic respiratory failure: CRF; cardiac failure: CF; arterial hypertension: AH; type 2 diabetes: DT2; continuous positive airway pressure: CPAP; partial O2 pressure in arterial blood: PaO2; fraction of inspired oxygen: FiO2.
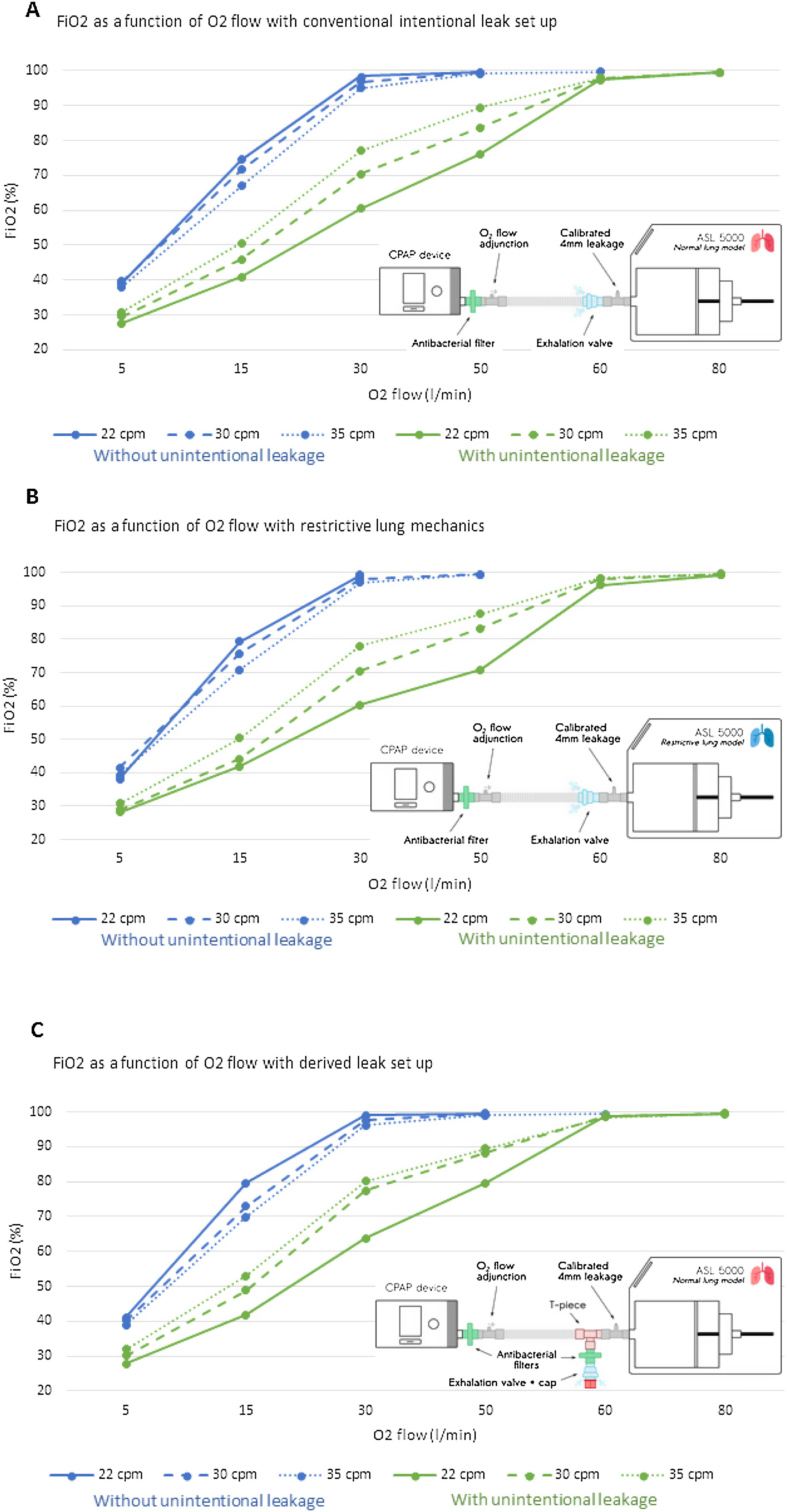
Fig. 1 shows the relationship between the O2 flow rate delivered and the resulting FiO2 in the simulation. Without unintentional leaks, a flow rate of at least 30L/min was necessary to reach FiO2>95%. FiO2 differed significantly for each respiratory rate at each O2 flow rate with and without unintentional leaks (p<0.001). With unintentional leaks, O2 flow rates of up to 80l/min were required to reach 100% FiO2 (Fig. 1A–C).
Dose–response relationship between oxygen (O2) flow rates delivered into the circuit, and fraction of inspired oxygen (FiO2) as a function of respiratory rate, presence/absence of non-intentional leakage, lung mechanics and leak port locations. Each Plot represents the mean FiO2 of the 10 cycles analysed. For each FiO2, the standard deviation was systematically below 0.3 and is therefore not represented on the graph. Panel A displays the results for the conventional intentional leak model, in which the unintentional leakage port was placed in series between the exhalation valve (intentional leak port) and the ASL 5000. Panel B displays the results for the same model as in panel A, but with restrictive lung mechanics. Panel C displays the results for by-pass leakage ports using an antibacterial filter between the ASL 5000 and both intentional and non-intentional leak ports. The blue lines represent the relationship between O2 flow and FiO2 without unintentional leaks, and the green lines represent the relationship between O2 flow and Fi2O with unintentional leaks.
The results of this experimental bench study confirmed our clinical observation that high O2 flow rates are required to achieve a high FiO2 in patients with severe Covid-19 using CPAP and vented masks. An O2 flow rate of at least 30L/min, and up to 80L/min, was required to achieve 100% FiO2 for all three experimental models. Reduced compliance and unintentional leakage dramatically reduced FiO2. The arbitrary compliance of 30ml/cmH2O used in model B is similar to that of patients with severe COVID-19.12 Such a low level of compliance would reduce the FiO2 with home CPAP and a vented mask, further confirming the need for high O2 flow rates. In our clinical cohort, three patients required an O2 flow rate above 80L/min: this very high rate causes two concerns. Firstly, we did not know the lung compliance of these patients. Secondly, the O2 flow rate required to achieve a given FiO2 is likely to be underestimated with the experimental model compared to the clinical situation, since no gas exchange occurs with an artificial lung. For instance, in a patient, with a 21% FiO2, the partial pressure of O2 in the inspired air will be 160mm Hg, while in the expired air (at sea level) it will be 120mm Hg due to oxygen absorption. Thus, in in vivo conditions even higher O2 flows than those reported in this study may be required.13
Another important point is that many teams use helmets to ventilate patients with Covid-19.2,14 Helmets could be more advantageous than oronasal masks because of the lower rate of unintentional leakage; this would allow lower oxygen flow rates to be used to achieve sufficient FiO2 levels for patients with severe hypoxemia.
Studies have shown that patients who cannot be intubated require a high FiO2 to maintain their SpO2 above 92%,3,15 and that poor oxygenation is strongly associated with mortality.3,16 Our study provides valuable data to guide clinicians in the use of O2 when using home CPAP in patients with severe Covid-19 who cannot be intubated. The results showed that these patients can be successfully treated with home CPAP devices, or NIV devices in CPAP mode, with vented masks, if a high O2 flow rate is provided. However, close monitoring of unintentional leaks is crucial to ensure a sufficient FiO2 for the treatment of severe hypoxemia in these patients.
Authors’ contributionAll authors participated in the study design, research, and manuscript preparation. Dr. Marius Lebret is the guarantor of the content of the manuscript, including the data and analysis.
FundingFunding was provided by Asten Santé.
Conflict of interestML reports receiving personal fees from Air Liquid Medical Systems and non-financial support from NOMICS over the past 3 years, for work unrelated to the work presented here. ML is a part-time employee of Air Liquide Medical System (Med2lab).
YC, CM and GP work as consultants for Air Liquid Medical System.
Dr. Marius Lebret is the guarantor of the content of the manuscript, including the data and analysis.
The authors would like to thank Asten Santé for providing funding the trial. The authors would also like to thank Johanna Robertson for English editing.