This report describes the general characteristics, objectives, and organizational aspects of the registries of minority respiratory diseases included in the National Registry of Rare Diseases of the Research Institute for Rare Diseases (ISCIII), in order to publicize their existence and encourage the participation of professionals.
Information is collected on the following conditions: alpha-1 antitrypsin deficiency, idiopathic tracheal stenosis, adult pulmonary Langerhans’ cell histiocytosis, lymphangioleiomyomatosis, alveolar proteinosis, and sarcoidosis.
En el presente trabajo se describen las características generales, objetivos y aspectos organizativos de los registros de enfermedades respiratorias minoritarias integrados en el Registro Nacional de Enfermedades Raras del Instituto de Investigación de Enfermedades Raras (IIER), con el objetivo de dar a conocer su existencia y fomentar la participación de los profesionales.
Se recoge información sobre registros de las siguientes enfermedades: déficit de alfa-1 antitripsina, estenosis traqueal idiopática, histiocitosis pulmonar de células de Langerhans del adulto, linfangioleiomiomatosis, proteinosis alveolar y sarcoidosis.
The Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) has a long and extensive experience in respiratory disease registries, including some minority respiratory diseases (MRD).1 The Instituto de Salud Carlos III (ISCIII) also included rare diseases (RD) as one of these priority areas of intervention and, as a result of this interest, the Research Institute for Rare Diseases (IIER) was created in 2003. The aim was to promote and conduct basic and clinical research, training and support in healthcare referral and innovation in the medical care of RD.
The functions of the IIER include the identification of the magnitude of RDs, establishing an epidemiological database for the retrieval of reliable information on the prevalence of these diseases and the distribution of healthcare resources allocated to their treatment. For this reason, in 2005 the National Rare Diseases Registry (RNER) was created to follow-up, monitor and conduct research into patients with RDs, their family members and control populations participating in research studies. The registry has been supported by the Spanish Rare Diseases Registry Research Network (Spain RDR: https://spainrdr.isciii.es), funded by the ISCIII as a network project within the International Rare Diseases Research Consortium (IRDiRC) (http://www.irdirc.eu; http://ec.europa.eu/research/health/medical-research/rare-diseases/irdirc_en.html).
The common objectives of both institutions were the driving force behind an agreement for the development of MRD registries in 2011, under which a project monitoring or steering group was created, each registry was assigned a leader, and SEPAR appointed a coordinator to oversee the progress made in the context of the agreement. The leaders of each MRD under the RNER coordinate the expert network for that disease. Functions include the design of variables specific to the characteristics and evolution of the entity and the promotion of research.
To optimize the task of coordinating disease databases within the RNER framework, the leaders of each registry and the contributing pulmonologists follow a set of internal regulations based on the criteria established by SEPAR (February 2013) and the recommendations of the IIER itself, in compliance with Spanish Data Protection Agency (AEPD) level 3 archive security. The conditions for use of these data are also described under this regulation.
National Rare Diseases RegistryThe RNER has 2 main strategic approaches: registries of patients based on clinical results and natural history, and population registries based on epidemiological and social welfare research and social welfare planning.
Patient registries may be created by agreement with scientific societies or based on the experience of a particular institution or expert network.1 One of the problems of this type of registry and the information they provide is that the cases are limited to those seen in participating centers, leaving open the possibility of a selection bias toward more severe cases. Notification of cases is voluntary, limited to some experts, and requires patient consent.2
At present, in addition to the SEPAR, the following scientific societies have reached a collaborative agreement with the ISCIII for participation in the strategy for development of patient registries within the RNER and SpainRDR: Spanish Society of Pediatric Pulmonology (SNEP), Spanish Society of Family and Community Medicine (semFYC), European Network of Rare and Congenital Anemias (ENERCA), Spanish Society of Allergy and Clinical Immunology (SEAIC), Spanish Society of Pediatric Endocrinology (SEEP), Spanish Society of Neurology (SEN) and Center of Energy, Environmental and Technological Research (CIEMAT). Negotiations are currently ongoing with another 5 societies and with other research networks such as CIBERNED. Agreements have also been signed with the pharmaceutical industry and the Spanish Federation of Rare Diseases (FEDER) itself. On an international level, the IRDiRC consortium has already set in motion projects closely related with SpainRDR, such as the RD-CONNECT project (http://www.rd-connect.eu). DG SANCO of the European Commission has already approved the EPIRARE project (http://www.epirare.eu). The aim of the former will be to standardize patient registries, biobanks and data from “omics” research on an international level, while the latter has set down the terms for the future European platform of RD registries that will be implemented in coming years in the European Commission Joint Research Center (JRC) in Ispra, Italy. The IIER is a member of both projects and is responsible for aspects such as defining the quality, selection and definition of common variables for interchange and interoperability among biobanks.
With regard to the functioning of the RNER and the SpainRDR Project, the population registry works with all the autonomous communities of Spain to collect information on all RDs. To this end, strategies have been developed for collecting data from various electronic sources such as the Minimum Basic Data Set (CMBD) that contains information on hospital discharge reports, death certificates, primary care sources, neonatal and congenital malformation screening programs, etc. In the near future, this information, once validated and consolidated, will allow us to estimate the incidence of the various diseases, regional distribution, prevalence and survival, depending on the quality of follow-up in all cases.3
Each of the 17 Spanish autonomous communities is setting up its own regional RD registry; in the autonomous cities of Ceuta and Melilla, the information is remitted to the IIER, and this organization will be responsible for setting up their corresponding registries. By way of these registries, each of the autonomous communities will supply the data derived from the various sources to the RNER.
The RNER also offers patients the possibility to self-register, that is to say, any individual with a disease classified as rare or his/her parents or legal guardians can register on the website, provide clinical information on their disease and give informed consent for the use of their data in research. The RNER can be accessed at https://registroraras.isciii.es.
To create MRD registries within the National Registry, each consultant committee has identified the relevant variables for data collection. In addition, the recording of common parameters has been standardized (demographic data, smoking, respiratory function tests, blood gases and the walking test) so that in the future data can be compared between registries. Furthermore, generic quality of life questionnaires have been added to the purely respiratory parameters to allow the impact of MRDs to be compared with other non-respiratory RDs or even with common diseases.
All the registries include quality data related to each condition and comply with the applicable data protection legislation. They will be accessible from the national registry website (https://registroraras.isciii.es), as well as from the SEPAR website (www.separ.es) or from the website of the SpainRDR project (https://spainrdr.isciii.es).
Each consultant committee is responsible for updating the contents of each registry, and the final responsibility lies with each coordinator, as representative of the consultant committee before SEPAR and the IIER.
The RNER itself does not provide funds for conducting parallel research based on registry data. This type of initiative must be funded, if required, by the societies themselves or national or international research agencies. The inclusion of the RNER in the international IRDiRC consortium will facilitate the creation of partnership agreements with similar registries in other countries.
This brief description gives an idea of the multiple applications of the available information and the advantages of accumulating quality data for biomedical research at a time in which current technological possibilities for sharing knowledge only serve to reinforce the individual experience of professionals in the interests of improving knowledge in these areas.
Spanish Registry of Patients With Alpha-1 Antitrypsin DeficiencyAlpha-1 antitrypsin (AAT) deficiency is the result of mutations that change the structure of the AAT protein, thus affecting its function. It appears mainly in the form of pulmonary emphysema, liver cirrhosis, and less frequently as other diseases (panniculitis, vasculitis, etc.).4 There are estimated to be around 12000 individuals with severe AAT deficiency in Spain.5
The Spanish Registry of Patients With Alpha-1 Antitrypsin Deficit (REDAAT) set up at the end of the 90s is part of the SEPAR chronic obstructive pulmonary disease (COPD) working area. Since it was founded, REDAAT has worked to further knowledge on AAT deficiency and awareness of this entity, improve treatment of affected subjects and encourage research.6–11
At present, REDAAT resources include a consultant committee consisting of 12 pulmonologists, 3 pediatrics and 3 basic researchers, in addition to reference laboratory managers and computer support staff. Over 300 physicians throughout Spain collaborate with this registry. The main technical resource is www.redaat.es, legally part of the Respira Spanish Lung Foundation. It has a public access area providing general information and a restricted access area for healthcare professionals where the form for recording patient data and real-time information on cases registered, overall characteristics, diagnoses and treatment can be found.
REDAAT has collaborated actively in the Alpha One International Registry ([AIR] http://www.antitrypsindeficiency.org) since its creation, as well as with some other European national registries.12,13
Since this registry has its own domain and an up-and-running database, created prior to the formation of the RNER, it is not integrated in this registry's platform although they do share their data.
A summary of cases registered is shown in Table 1.
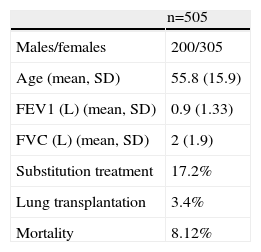
Basic Population Data of Spanish Registry of Patients With Alpha-1 Antitrypsin Deficiency Patients (2001–2013).
| n=505 | |
| Males/females | 200/305 |
| Age (mean, SD) | 55.8 (15.9) |
| FEV1 (L) (mean, SD) | 0.9 (1.33) |
| FVC (L) (mean, SD) | 2 (1.9) |
| Substitution treatment | 17.2% |
| Lung transplantation | 3.4% |
| Mortality | 8.12% |
SD: standard deviation; FEV1: forced expiratory volume in first second; FVC: forced vital capacity; L: liters.
Two groups can defined under the term tracheal stenosis: secondary benign laryngotracheal stenosis and idiopathic tracheal stenosis.14
Idiopathic tracheal stenosis is a disease characterized by the development of fibrous stenotic scarring in the cricoid region and the upper third of the trachea, with thickening of the submucosa but no underlying cartilage involvement. It mainly affects women, and the principal symptoms are dyspnea and stridor developing over a period of years. Diagnosis is incompatible with a history of other upper airway stenosis that could be defined as secondary.15
Incidence and prevalence are unknown, as are etiology and prognostic factors. No specific diagnostic and treatment guidelines are available and it has not yet been included in international RD catalogs. Accumulated experience with this disease is centered mainly on the various therapeutic surgical techniques.16–21
Secondary benign laryngotracheal stenosis is usually due to one of the following causes: post-intubation, post-trauma, inhalation of toxic smoke, post-surgery, infection, inflammatory activity, extrinsic and neurological compression, and are not the focus of this registry.14
In the last 30 years, the development of tracheal surgery, along with other therapeutic alternatives (laser, dilations and tracheal prosthetic devices), has meant that most of these benign laryngotracheal lesions can be treated, although accumulation of experience in healthcare centers is limited by their relative rarity.
The Spanish Tracheal Stenosis Registry (REET) is an integral part of the SEPAR Thoracic Surgery Area. Over 40 professionals have joined this registry as collaborators, its database is an integral part of the RNER platform, and data collection will begin in 2014.
Spanish Registry of Pulmonary Langerhans Cell HistiocytosisPulmonary Langerhans cell histiocytosis (PLCH) in adults is characterized by a peribronchiolar proliferation of Langerhans histiocytes, a dendritic cell involved in the immune response. It primarily affects adults between 20 and 40 years of age, with no preference for sex. Progress is generally benign, although around 30% of patients have progressively deteriorating lung function. It is associated with smoking, with 95% of patients being smokers, but etiology is unknown and the exact incidence and prevalence of PLCH in adults has not been determined.
An expert consensus paper was recently published giving recommendations on the diagnosis and treatment of this disease that is hoped to go some way toward standardizing procedures.22 The main treatment in severe cases is lung transplantation, although the use of chemotherapeutic agents for treating progressive disease has been proposed.
There are very few data in our setting on the characteristics and management of PLCH. The first multicenter series was published recently, showing the importance of the use of clinical guidelines in the diagnosis and treatment of the disease, as mentioned above.23 Moreover, the question of tobacco as a triggering factor in the disease and other potential physiopathological pathways need to be explored in further depth.
The Spanish Registry of Pulmonary Langerhans Cell Histiocytosis (REHPCL) is part of the SEPAR diffuse interstitial disease integrated research program (PII-EPID). It consists of a consultant committee of 5 experts and the database is hosted on the RNER platform. Data collection began in September 2013. This registry, in addition to its purely epidemiological aspect, aims to develop parallel translational research projects in this disease, and launched the PLCH Functional and Genetic Characterization project, in which several SEPAR and IIER (ISCIII) investigators are actively involved. The project has been funded by a grant from the Fundación Mútua Madrileña and the PI-EPID (EPID-Futuro Rech Assistance).
Spanish Lymphangioleiomyomatosis RegisterLymphangioleiomyomatosis (LAM) is a lung disease affecting young women of childbearing potential. It is characterized by abnormal growth of atypical smooth muscle cells (LAM cells) in the lung, including the airways and the lymphatic and blood vessels, leading to cyst formation and finally the destruction of the lung parenchyma. Two types of LAM are distinguished: a sporadic form with exclusively pulmonary involvement (sporadic LAM) and another associated with tuberous sclerosis complex, a dominant autosomic neurocutaneous syndrome linked with TSC1 and TSC2 gene mutations.
Due to its low prevalence, this disease is relatively unknown. Many of the symptoms (dyspnea, cough) are similar to those of other lung diseases such as asthma or chronic bronchitis, so diagnosis is generally delayed.
The true incidence of LAM is unknown. In the United States, 1300 patients are registered in the American LAM Foundation. In a cross-sectional study carried out in Spain, the SEPAR Diffuse Interstitial Pulmonary Disease (EPID) working group in collaboration with the Spanish LAM Patients Association (AELAM) registered 72 cases from 23 centers in 8 autonomous communities plus Andorra, forming the basis of the Spanish LAM Registry (RELAM). The general characteristics of this registry are described in Table 2.24
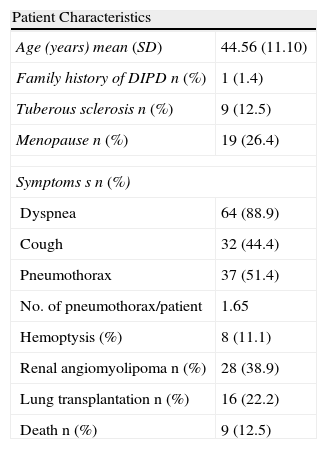
Characteristics of LAM Patients.
| Patient Characteristics | |
| Age (years) mean (SD) | 44.56 (11.10) |
| Family history of DIPD n (%) | 1 (1.4) |
| Tuberous sclerosis n (%) | 9 (12.5) |
| Menopause n (%) | 19 (26.4) |
| Symptoms s n (%) | |
| Dyspnea | 64 (88.9) |
| Cough | 32 (44.4) |
| Pneumothorax | 37 (51.4) |
| No. of pneumothorax/patient | 1.65 |
| Hemoptysis (%) | 8 (11.1) |
| Renal angiomyolipoma n (%) | 28 (38.9) |
| Lung transplantation n (%) | 16 (22.2) |
| Death n (%) | 9 (12.5) |
SD: standard deviation; DIPD: diffuse interstitial pulmonary disease; LAM: lymphangioleiomyomatosis; NK/NA: not known/not applicable.
Source: Adapted from Antón et al.23
RELAM is an integral part of the PI-EPID and has an 8-member consultant committee. The database is hosted on the RNER platform and data collection started in September 2013.
Spanish Alveolar Proteinosis RegistryPulmonary alveolar proteinosis (PAP) is a disease characterized by the accumulation of proteins and surfactant lipids in the alveoli, causing gas exchange problems that can trigger respiratory insufficiency.
There are 3 types of PAD: autoimmune or acquired, secondary and congenital.
The autoimmune type accounts for 90% of PAP in adults. It generally occurs in subjects around 50–60 years of age, and more often in men (2:1). It presents with anti-granulocyte-macrophage colony stimulating factor antibodies (anti-GM-CSF). The secondary type, that accounts for the remaining 10% of PAP in adults, presents at a similar age as the autoimmune type and is also more common in men (1.5:1) with hematological diseases (particularly myelodysplastic syndrome), respiratory infections or autoimmune diseases. Anti-GM-CSF antibodies are negative and prognosis is worse than for the autoimmune type (mean 2-year survival of 50% and 5-year survival of 35%). Lastly, the congenital type is known to be associated with genetic mutations coding for lung surfactant proteins.
The general prevalence of PAP is 3.7 patients per million inhabitants, although this depends to some extent on the geographical regions, due to association with race.
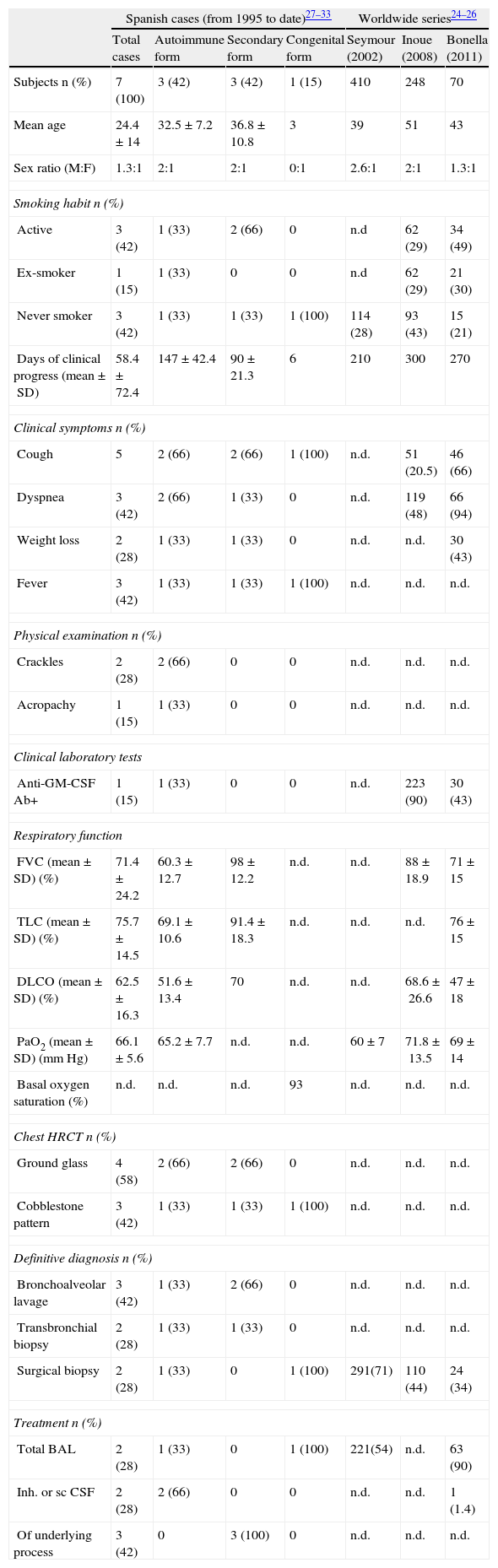
The total number of cases in Spain is unknown. The most significant results from national and international series are summarized in Table 3.25–34
Clinical Data from Cases of PAP Published in Spain to Date Since 1995, Grouped According to Etiology. Summary of Large Worldwide PAP Series Published To Date.
| Spanish cases (from 1995 to date)27–33 | Worldwide series24–26 | ||||||
| Total cases | Autoimmune form | Secondary form | Congenital form | Seymour (2002) | Inoue (2008) | Bonella (2011) | |
| Subjects n (%) | 7 (100) | 3 (42) | 3 (42) | 1 (15) | 410 | 248 | 70 |
| Mean age | 24.4±14 | 32.5±7.2 | 36.8±10.8 | 3 | 39 | 51 | 43 |
| Sex ratio (M:F) | 1.3:1 | 2:1 | 2:1 | 0:1 | 2.6:1 | 2:1 | 1.3:1 |
| Smoking habit n (%) | |||||||
| Active | 3 (42) | 1 (33) | 2 (66) | 0 | n.d | 62 (29) | 34 (49) |
| Ex-smoker | 1 (15) | 1 (33) | 0 | 0 | n.d | 62 (29) | 21 (30) |
| Never smoker | 3 (42) | 1 (33) | 1 (33) | 1 (100) | 114 (28) | 93 (43) | 15 (21) |
| Days of clinical progress (mean±SD) | 58.4±72.4 | 147±42.4 | 90±21.3 | 6 | 210 | 300 | 270 |
| Clinical symptoms n (%) | |||||||
| Cough | 5 | 2 (66) | 2 (66) | 1 (100) | n.d. | 51 (20.5) | 46 (66) |
| Dyspnea | 3 (42) | 2 (66) | 1 (33) | 0 | n.d. | 119 (48) | 66 (94) |
| Weight loss | 2 (28) | 1 (33) | 1 (33) | 0 | n.d. | n.d. | 30 (43) |
| Fever | 3 (42) | 1 (33) | 1 (33) | 1 (100) | n.d. | n.d. | n.d. |
| Physical examination n (%) | |||||||
| Crackles | 2 (28) | 2 (66) | 0 | 0 | n.d. | n.d. | n.d. |
| Acropachy | 1 (15) | 1 (33) | 0 | 0 | n.d. | n.d. | n.d. |
| Clinical laboratory tests | |||||||
| Anti-GM-CSF Ab+ | 1 (15) | 1 (33) | 0 | 0 | n.d. | 223 (90) | 30 (43) |
| Respiratory function | |||||||
| FVC (mean±SD) (%) | 71.4±24.2 | 60.3±12.7 | 98±12.2 | n.d. | n.d. | 88±18.9 | 71±15 |
| TLC (mean±SD) (%) | 75.7±14.5 | 69.1±10.6 | 91.4±18.3 | n.d. | n.d. | n.d. | 76±15 |
| DLCO (mean±SD) (%) | 62.5±16.3 | 51.6±13.4 | 70 | n.d. | n.d. | 68.6±26.6 | 47±18 |
| PaO2 (mean±SD) (mmHg) | 66.1±5.6 | 65.2±7.7 | n.d. | n.d. | 60±7 | 71.8±13.5 | 69±14 |
| Basal oxygen saturation (%) | n.d. | n.d. | n.d. | 93 | n.d. | n.d. | n.d. |
| Chest HRCT n (%) | |||||||
| Ground glass | 4 (58) | 2 (66) | 2 (66) | 0 | n.d. | n.d. | n.d. |
| Cobblestone pattern | 3 (42) | 1 (33) | 1 (33) | 1 (100) | n.d. | n.d. | n.d. |
| Definitive diagnosis n (%) | |||||||
| Bronchoalveolar lavage | 3 (42) | 1 (33) | 2 (66) | 0 | n.d. | n.d. | n.d. |
| Transbronchial biopsy | 2 (28) | 1 (33) | 1 (33) | 0 | n.d. | n.d. | n.d. |
| Surgical biopsy | 2 (28) | 1 (33) | 0 | 1 (100) | 291(71) | 110 (44) | 24 (34) |
| Treatment n (%) | |||||||
| Total BAL | 2 (28) | 1 (33) | 0 | 1 (100) | 221(54) | n.d. | 63 (90) |
| Inh. or sc CSF | 2 (28) | 2 (66) | 0 | 0 | n.d. | n.d. | 1 (1.4) |
| Of underlying process | 3 (42) | 0 | 3 (100) | 0 | n.d. | n.d. | n.d. |
SD: standard deviation; DLCO: carbon monoxide diffusion capacity; FVC: forced vital capacity; GM-CSF: granulocyte-monocyte colony stimulating factor; inh: inhaled; BAL: bronchoalveolar lavage; n.d.: no data; PaO2: arterial oxygen pressure; PAP: pulmonary alveolar proteinosis; Sat: saturation; sc: subcutaneous; HRCT: high resolution computed tomography; TLC: total lung capacity.
The Spanish Alveolar Proteinosis Registry (REPA) is an integral part of the SEPAR PII-EPID and its consultant committee consists of 5 pulmonologists. The database is hosted on the RNER platform and cases have been registered since September 2013.
Spanish Sarcoidosis RegistrySarcoidosis is a systemic granulomatosis disease affecting mainly the lung (90%) and lymphatic tissue, but other organs can also be involved.35
Overall incidence and prevalence vary depending on the geographical region analyzed: over 50/100000 cases are reported in Nordic countries, while in others such as Japan or Spain, prevalence of <10/100000 inhabitants is reported, among which Löfgren syndrome is very common (48%).36 It mainly affects middle-aged adults. Large series of patients studied in the last 30 years suggest that a combination of predisposing environmental factors and a genetic makeup susceptible to developing the disease are necessary, but no conclusive studies have been published.37,38 Several papers published point to the relevance of certain infectious agents (bacteria, viruses and mycoses) and exposure to rural environments.
One of the main clinical problems is to determine the existence of inflammatory activity. In this respect, angiotensin-converting enzyme (ACE) levels have been studied in most depth, although hyposensitivity in delayed cutaneous hypersensitivity tests and lymphopenia have also been used.39 Patients with this disease have also been seen to have immunological dysfunction in macrophage and T-lymphocyte recognition, processing and presentation of potential antigens.40
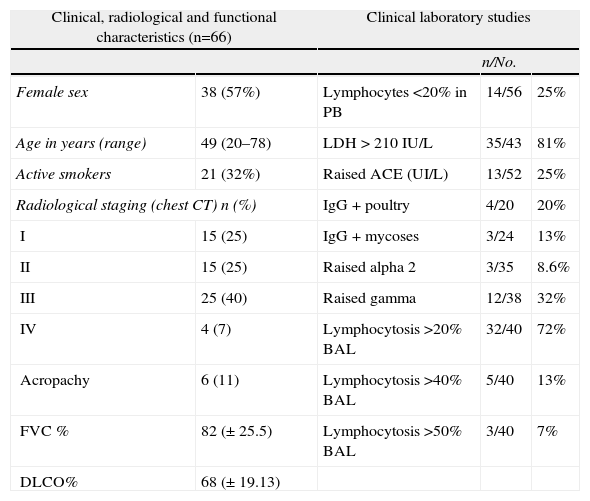
The general data from the largest series published in Spain are given in Table 4, and the characteristics of the series from the Hospital Vall d’Hebron (2005–2012) that was the starting point of this registry are presented in Table 5.41
Sarcoidosis Series From the Hospital Universitario Vall d’Hebron (2004–2012).
| Clinical, radiological and functional characteristics (n=66) | Clinical laboratory studies | |||
| n/No. | ||||
| Female sex | 38 (57%) | Lymphocytes<20% in PB | 14/56 | 25% |
| Age in years (range) | 49 (20–78) | LDH>210IU/L | 35/43 | 81% |
| Active smokers | 21 (32%) | Raised ACE (UI/L) | 13/52 | 25% |
| Radiological staging (chest CT) n (%) | IgG+poultry | 4/20 | 20% | |
| I | 15 (25) | IgG+mycoses | 3/24 | 13% |
| II | 15 (25) | Raised alpha 2 | 3/35 | 8.6% |
| III | 25 (40) | Raised gamma | 12/38 | 32% |
| IV | 4 (7) | Lymphocytosis>20% BAL | 32/40 | 72% |
| Acropachy | 6 (11) | Lymphocytosis>40% BAL | 5/40 | 13% |
| FVC % | 82 (±25.5) | Lymphocytosis>50% BAL | 3/40 | 7% |
| DLCO% | 68 (±19.13) | |||
Raised alpha 2: >10.7%; DLCO: carbon monoxide diffusion capacity; ACE: angiotensin-converting enzyme; FVC: forced vital capacity; raised gammaglobulin: >19.3%; IgG: immunoglobulin G; BAL: bronchoalveolar lavage; LDL: low density lipoprotein; PB: peripheral blood; CT: computed tomography.
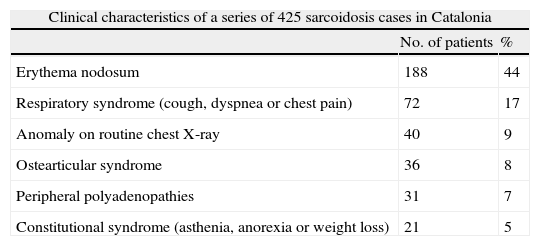
Sarcoidosis in Catalonia: Analysis of 425 Cases.
| Clinical characteristics of a series of 425 sarcoidosis cases in Catalonia | ||
| No. of patients | % | |
| Erythema nodosum | 188 | 44 |
| Respiratory syndrome (cough, dyspnea or chest pain) | 72 | 17 |
| Anomaly on routine chest X-ray | 40 | 9 |
| Ostearticular syndrome | 36 | 8 |
| Peripheral polyadenopathies | 31 | 7 |
| Constitutional syndrome (asthenia, anorexia or weight loss) | 21 | 5 |
The Spanish Sarcoidosis Registry (RESAR) is an integral part of the PI-EPID and its consultant committee consists of 7 physicians. The database is hosted on the RNER platform and cases have been registered since September 2013.
Although the registries were initially developed by a small group of experts in each disease, they represent a collaborative tool in which any clinician can report a case and any investigator can approach the scientific committee for permission to exploit the registered data.
DiscussionThis article gives information on the MRD registries developed in Spain in coordination with the RNER.
The data gathered from the few existing cases will allow the scientific community to gain deeper insight into these diseases, and it will also be of use to non-expert clinicians who, if they come across a patient with one of these rare disorders, can avail themselves of this up-to-date information and gain access to expert advice. Access to updated and dynamic data from all over Spain will also be invaluable in helping healthcare institutions allocate care resources for patients with these rare conditions.
Incorporation of the information in the population-based registries of each autonomous community in Spain will give a better overview of the reality of daily clinical practice than can be gleaned from voluntary registries.
Like clinical trials, quality data are essential for obtaining valid results. Being observational studies, registries are exposed to bias of many kinds, although the most common are those affecting selection of cases and the quality of the available data, particularly with regard to the principal variables (accurate diagnosis and coding).42 In the RNER, data from patient registries can be compared with those contributed by the autonomous community registries, meaning that the representativeness of the sample and thus the validity of the results can be determined.
The role of registries in decision-making with regard to the efficacy of treatments remains controversial. Nevertheless, when they contain a larger number of cases with more adverse events than a clinical trial, their role in the post-approval monitoring of side effects and in the cost-benefit analysis of these treatments is essential, and cannot be replaced with the traditional clinical trial design.43–45
The RNER of the IIER (ISCIII) forms part of the Rare Diseases Strategy of Spanish National Health System. It plays a key role in this strategy by acting as a source of information for the development and planning of social welfare policies and for research. Contributions from all the autonomous communities, together with input from professional societies, industry and patients themselves, make these registries a unique tool for maximizing synergies and provide enormous scope for international cooperation.
Authorship/CollaborationBeatriz Lara: preparation of the article and REDAAT administrator; Manuel Posada and Ignacio Abaitua: preparation of the article and responsible for the design and setting up of the RNER; Genaro Galán: preparation of the article and REET coordinator; Diego Castillo: preparation of the article and REHPCL coordinator; Álvaro Casanova: preparation of the article and RELAM coordinator; Esteban Cano: preparation of the article and REPA coordinator; Íñigo Ojanguren: preparation of the article and RESAR coordinator.
Conflict of InterestThe authors declare that they have no conflict of interest and have participated equally in the preparation of this paper.
The authors would like to thank the Spanish Association of Patients with Alpha-1 Antitrypsin Deficiency, represented by Mrs Shane Fitch and Mr Mariano Pastor, and the Spanish Association of Patients with Lymphangioleoimyomatosis, represented by Mrs Asunción Valdivielso and Mrs María Luz Vila, for their extraordinary work.
They also thank Dr Ruiz Manzano, SEPAR Chairman in 2011, for his invaluable support throughout the project, without whom it would not have been possible.
Please cite this article as: Lara Gallego B, Abaitua Borda I, Galán Gil G, Castillo Villegas D, Casanova Espinosa Á, Cano Jiménez E, et al. Registros de enfermedades respiratorias integrados en el Registro Nacional de Enfermedades Raras. Arch Bronconeumol. 2014;50:397–403.