D-dimer levels are increased in pulmonary embolism (PE) but also in many other conditions including inflammation, cancer, pregnancy, trauma, and sepsis.1 D-dimer is a useful rule-out test for avoiding imaging in several clinical settings.2 In fact, in patients with low or intermediate clinical probability, D-dimer has negative predictive value to exclude deep vein thrombosis or PE without further testing in the outpatient setting.3 Nevertheless, its usefulness in hospitalized patients with suspected thromboembolism is less well established. Only few studies have evaluated the predictive value of quantification of D-dimers in hospitalized patients with PE,4 and there are no studies addressing this topic in patients with COVID-19. Thus, in the context of the COVID pandemic, in which seriously ill patients have respiratory symptoms, it is even more convenient to find an adequate value of D-dimer that can help when requesting imaging studies, such as computed tomography pulmonary angiography (CTPA).
A retrospective study was performed to analyze the predictive value of D-dimer to assess CTPA for diagnosis of PE in patients with COVID-19 pneumonia during their hospitalization. The local Clinical Research Ethical approved the study.
All patients included in current study were COVID-19 positive according to present diagnostic criterion.5 They had undergone CTPA scans due to suspected PE and underwent D-dimer tests according guidelines.6 D-dimers were checked at least at the time of admission and prior to CTPA. D-dimer (local reference range: <500mcg/L FEU), was measured by a commercial latex-enhanced immunoturbidimetric assay (Siemens AG SYSMEX CS-5100). CTPA examinations were obtained in a multidetector CT scanner (Discovery CT750 HD, GE Healthcare, Milwaukee, USA) by using a Dual-Energy CTPA protocol (Gemstone Spectral Imaging GSI). Highest observed values of D-dimer (of at least one assessment during hospitalization) before CTPA for each patient were used as diagnostic threshold and their sensitivity and specificity was estimated. Positive predictive (PPV)- and negative predictive (NPV)-values were calculated to evaluate the correct positive and correct negative test procedure results.7 The calculations were made with SPSS/PC for Windows (version 25.0, SPSS Inc., Chicago, IL, USA) and MedCalc (version 9.3.9.0; MedCalc, Mariakerke, Belgium). P values of <.05 were considered statistically significant.
A total of 52 patients with a confirmed diagnosis of COVID-19 pneumonia and suspected PE were included. The main causes for CTPA assessment were clinical worsening (85%) and/or elevated D-dimer (15%). Only two patients had right ventricle dilatation at the time of PE diagnosis. Dyslipidaemia and obesity were more frequent in patients with PE, but there were no significant differences found between groups when analysing with other variables. Forty-nine patients received low weight molecular heparin (LWMH) as thromboprophylaxis at standard dose (40mg/day, n=25) or intermediate dose (1mg/kg/day, n=18) according D-dimer value (≤2000 and >2000, respectively) or therapeutic LWMH (1mg/kg/12h, n=6) for medical conditions (atrial fibrillation and others). Three patients (2 with PE and 1 non-PE) did not receive thromboprophylaxis.
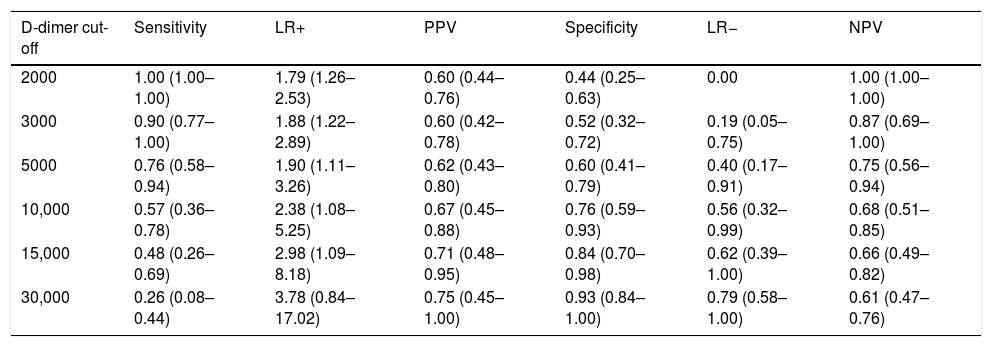
At the time of admission, D-dimer levels were not different among patients that developed PE [(median (P5–P75) 2350 (1070–10500)mcg/L] and those who did not [3030 (650–12415)mcg/L], (P=.87). We found significant differences in the highest values of D-dimer before performing CTPA only in patients with PE [14,240 (5140–31550)mcg/L, P=.007]. The mean changes from the baseline to the highest values before CTPA for patients with PE was 9406 (2917)mcg/L. In Table 1, we set out estimates of sensitivity, specificity, Positive Predicted Value (PPV), Negative Predicted Value (NPV), Positive Likelihood Ratio (LR+), and (LR−) values to predict the diagnosis of PE. D-dimer of 2000mcg/L resulted in the best cut-off point of sensitivity for patients with PE: sensitivity, 1.00; PPV, 0.60; specificity, 0.44; and, NPV, 1.00; LR−, 0. Using this threshold there were zero negative false cases; however, there were 18 (35%) positive false cases. By contrast, D-dimer of 30,000mcg/L or higher was the best threshold for the diagnosis of PE; sensitivity, 0.26; PPV, 0.75; specificity, 0.93; NPV, 0.61, and LR+, 3.78; but we found only 2 (3.8%) positive false cases at this cut-off. In addition, we found that a variation in D-dimer of 4000mcg/L or more from admission to the highest value before CPTA was predictive of PE with a sensitivity, 0.48; PPV, 0.79; specificity, 0.90; NPV, 0.68; LR+ 4.62, and LR− 0.58. This magnitude showed only 3 positive false cases. Among the subjects included in the study, only 2 deaths were confirmed to be caused by severe respiratory syndrome, with no evidence of PE.
Sensitivity, specificity, PPV, LR+, LR− and NPV with 95% confidence interval for highest levels of D-dimer (mcg/L) at different cut-off points for prediction pulmonary embolism in patients with COVID-19 during hospitalization.
| D-dimer cut-off | Sensitivity | LR+ | PPV | Specificity | LR− | NPV |
|---|---|---|---|---|---|---|
| 2000 | 1.00 (1.00–1.00) | 1.79 (1.26–2.53) | 0.60 (0.44–0.76) | 0.44 (0.25–0.63) | 0.00 | 1.00 (1.00–1.00) |
| 3000 | 0.90 (0.77–1.00) | 1.88 (1.22–2.89) | 0.60 (0.42–0.78) | 0.52 (0.32–0.72) | 0.19 (0.05–0.75) | 0.87 (0.69–1.00) |
| 5000 | 0.76 (0.58–0.94) | 1.90 (1.11–3.26) | 0.62 (0.43–0.80) | 0.60 (0.41–0.79) | 0.40 (0.17–0.91) | 0.75 (0.56–0.94) |
| 10,000 | 0.57 (0.36–0.78) | 2.38 (1.08–5.25) | 0.67 (0.45–0.88) | 0.76 (0.59–0.93) | 0.56 (0.32–0.99) | 0.68 (0.51–0.85) |
| 15,000 | 0.48 (0.26–0.69) | 2.98 (1.09–8.18) | 0.71 (0.48–0.95) | 0.84 (0.70–0.98) | 0.62 (0.39–1.00) | 0.66 (0.49–0.82) |
| 30,000 | 0.26 (0.08–0.44) | 3.78 (0.84–17.02) | 0.75 (0.45–1.00) | 0.93 (0.84–1.00) | 0.79 (0.58–1.00) | 0.61 (0.47–0.76) |
Definition of abbreviations: LR+, positive likelihood ratio; LR−, negative likelihood ratio; PPV, positive predicted value; NPV, negative predicted value.
The current retrospective study identified that a D-dimer value of 2000 mcg/L was the best sensitivity cut-off point to rule out PE in patients with COVID-19 pneumonia. Besides, we recognized a D-dimer level of 30,000mcg/L as the best value of specificity to predict PE. Also, an increase of D-dimer of 4000mcg/L from admission to the highest determination during hospitalization was found to be the best value to detect PE.
To our knowledge, this is the first report with the aim to identify specific D-dimer cut-off values in patients with COVID-19 pneumonia to predict PE. Several studies have shown that the risk of thrombotic events increases with rising D-dimer concentration in acutely ill-hospitalized patients.4,8 A recent study has demonstrated that despite systematic thrombosis prophylaxis, the incidence of thrombotic complications (mainly PE) in ICU patients with COVID-19 infections is notably high.9
It has been stated that viral infections could lead to a prothrombotic state. Dengue virus has been stated to give rise to increased Interleukin-6 (IL-6), which in turn leads to an increase in Plasminogen activator inhibitor-1 (PAI-1)10 a decrease in tPA which leads to a pro-thrombotic state. Likewise, severe cases of COVID-19 and original SARS virus were associated with an increase IL-6 level (and other inflammatory markers),11 and elevated PAI-1 levels,12 respectively.
Our study presents several limitations: its retrospective nature and a sample size is small. These shortcomings are however counterbalanced by two strengths. First, the wide range of D-dimer values both at admission and during hospitalization, second, 40% incidence when PE is suspected suggests a considerable frequent occurrence.
In conclusion, D-dimer monitoring helps in the evaluation of PE in patients with COVID-19 pneumonia and they should be considered in the clinical management of these patients. Our study opens the venue to future multicenter studies with a larger sample size for adequate validation.