Pulmonary arteriovenous malformations (PAVMs) are abnormal communications between an artery and a vein without an intervening capillary component.1•5 While most PAVMs are congenital, caused by hereditary hemorrhagic telangiectasia (also known as Osler-Weber-Rendu disease), approximately 20% are acquired and can be due to chronic liver disease, schistosomiasis, mitral stenosis, trauma, previous cardiac surgery, actinomycosis, Fanconi syndrome, tuberculosis or tumors.3,4,6 Tumors may be followed by the formation of PAVMs upon long-term complete remission with treatment, a phenomenon which therefore may especially occur in case of metastatic tumors that are amenable to curing using chemotherapy, i.e. choriocarcinoma. Here, we report a case of PAVM following metastatic gestational trophoblastic neoplasia (GTN).
We report a case of a 67-year-old woman, occasional cigar smoker, who during her first pregnancy in 1977, when she was 27, presented with a hydatidiform mole requiring several curettages. After an uterine rupture, a hysterectomy was performed. In the postoperative period, pulmonary metastases were detected, visualized as a perihilar left opacity. Chemotherapy with methotrexate and vincristine was then performed from April to June 1977, with a complete remission.
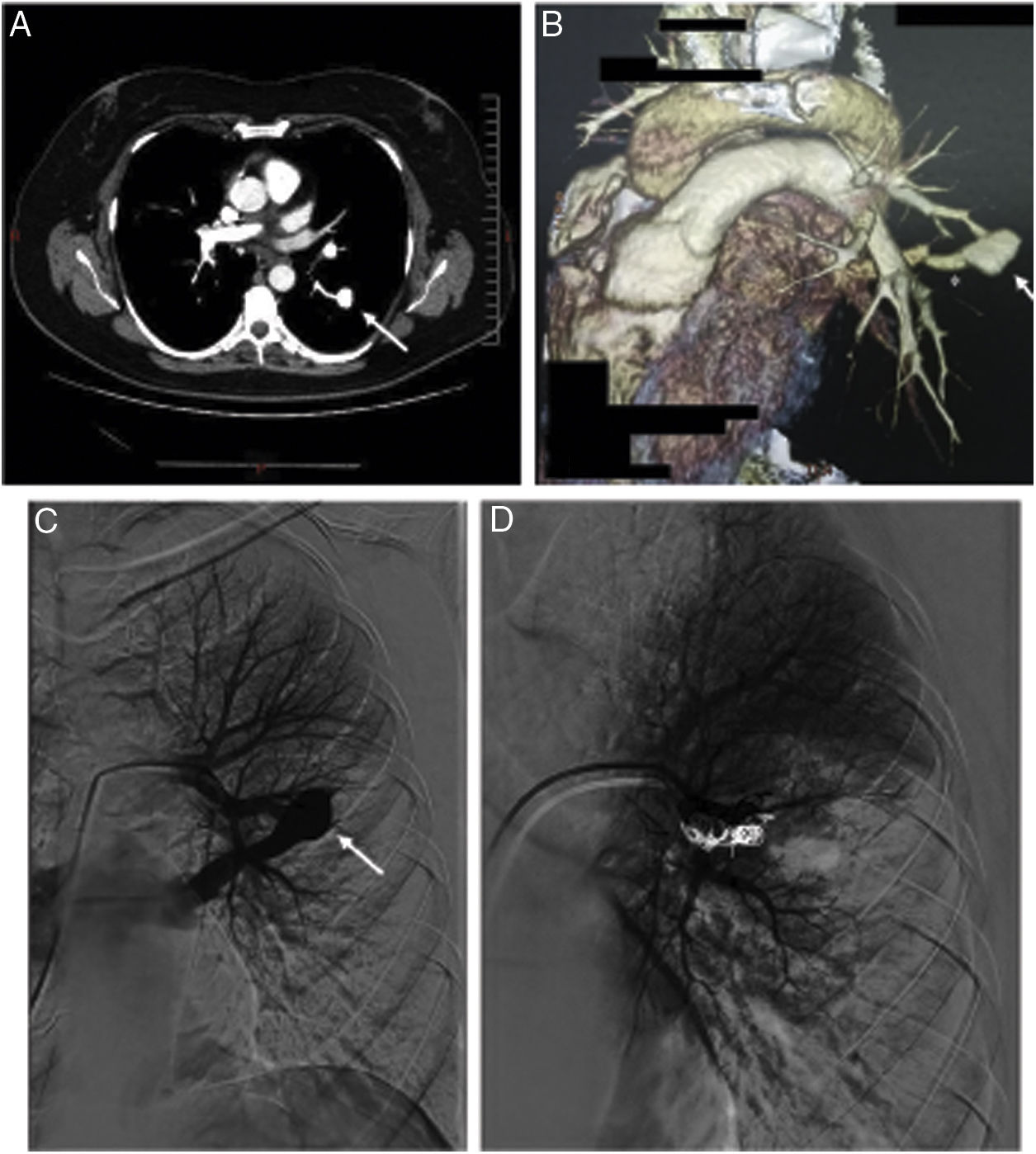
In December 2016, after an upper digestive endoscopy, a respiratory infection was diagnosed. In order to control the response to therapeutics, she underwent a chest radiography revealing a pulmonary nodule. The CT-scan showed a nodule, 20mm in diameter, located in the apical segment of the left lower lobe (Fig. 1). Upon contrast injection, on the arterial phase, a complete fill-in of this nodule was seen, which formed an aneurysmal sac, with a connection to a sub-segmental branch of the upper branch of the pulmonary artery, and to a pulmonary vein, at the lower end. No internal thrombosis was observed. The diagnosis of PAVM was made. The patient was asymptomatic and denied episodes of hemoptysis, trauma, thoracic surgery, and no personal nor family history of epistaxis. At examination, no telangiectasia was seen in the oral cavity, lips, face or fingertips. The pulmonary auscultation was normal, with no audible thoracic murmur. Peripheral oxygen saturation was normal, with 97% at resting and in room air. Human chorionic gonadotropin (hCG) and hCG beta chain were low (<1UI/L and <0.04ng/mL respectively). The echocardiography and spirometry were normal.
Thoracic CT scan and angiogram images of the pulmonary arteriovenous malformation. (A) The axial thoracic CT image (mediastinal window) shows an aneurysmal sac (arrow), with a connection to a sub-segmental branch of the upper branch of the pulmonary artery. (B) 3D-volume rendering CT image that shows an aneurysmal sac with a connection to a sub-segmental branch of the upper branch of the pulmonary artery, and at the lower end to a pulmonary vein (*). (C) Selective left pulmonary artery branch angiogram showing the pulmonary arteriovenous malformation (arrow). (D) Postembolization pulmonary arteriogram with complete occlusion of the pulmonary arteriovenous malformation.
Based on the history of molar pregnancy, and on the imaging demonstrating a typical PAVM in a territory where a metastasis of trophoblastic tumor had regressed with treatment, we diagnosed isolated PAVM developed on pulmonary sequelae of a metastatic molar pregnancy. The patient underwent percutaneous coil vaso-occlusion, with no complication and a good immediate angiographic result.
Molar pregnancies are classified as gestational trophoblastic disease, which include hydatidiform moles, invasive moles, choriocarcinomas, placental site trophoblastic tumors and epithelioid trophoblastic tumors.7 They occur at a rate of approximately one in every 600 conceptions.8 Approximately 10% of complete hydatidiform moles and 0.5% of partial hydatidiform moles undergo malignant transformation called GTN.5,7,8 In the majority of GTNs, the disease is limited to the uterus where the abnormal trophoblast proliferation and localized hCG production may lead to focal vascular changes, including the formation of AVMs.5,8 A recent systematic review of uterine AVMs following gestational trophoblastic neoplasia found 50 cases.5 Although the lung is the most frequent site of metastasis in GTN, the formation of PAVMs has been rarely described, and mostly in cases of choriocarcinoma.4,6,8,9
Although most patients are asymptomatic, PAVM can lead to dyspnea1 and complications such as hemoptysis, hemothorax,2 bacterial endocarditis,6 brain abscess or embolic events.2,3,6 Even though the natural history of PAVMs is not well known, morbidity and mortality of untreated PAVM are thought to be higher than those associated with treatment.6 PAVM can be treated either by surgery or by embolization but the latter has been recommended as treatment of choice.2,3 The possibility of recurrence due to recanalization occurring in up to 25% of cases is the major concern with embolization therapy.3
As far as we know, this is the second report of a PAVM after a gestational trophoblastic disease such as molar pregnancy. As GTN are highly vascularized tumors, due to high levels of HCG which stimulate angiogenesis,5,8 we hypothesize that a PAVM was formed within or next to the pulmonary metastasis of the GTN and remained at the metastatic site, even though the metastasis was successfully controlled by chemotherapy and no recurrence was found (proved by the low serum value of HCG). Such mechanism can only be hypothesized for metastasis from tumors which can be cured by chemotherapy. In fact, it has been described that AVM can persist after a successful eradication of the GTN by chemotherapy.1,4,6,9