Respiratory disease exacerbated by aspirin and other non-steroidal anti-inflammatory drugs (NSAIDS) is characterized by asthma, chronic rhinosinusitis, and nasal polyps aggravated by the administration of cyclooxygenase enzyme (COX)-1 inhibitors.
The prevalence among adult asthma patients is estimated at 7%–20%, and is even higher in patients with severe asthma. Symptoms usually begin in the third or fourth decade of life, more frequently in women.1 It is much more uncommon in children,2 and few cases have been reported in the literature to date.3–5
Children with this phenotype tend to have moderate-severe asthma, even when the administration of NSAIDs is avoided. When compared with other asthma phenotypes, patients with aspirin-exacerbated respiratory disease have more frequent exacerbations, need higher doses of inhaled (or in many cases systemic) corticosteroids for asthma control, and show greater spirometric involvement.6 Significant rhinosinusal involvement is another aggravating factor.
The underlying pathogenic mechanisms are dysregulation of arachidonic acid metabolism and greater activation of eosinophils, platelets, and mast cells, with increased proinflammatory prostaglandins caused by the administration of COX-1 inhibitors.7
Challenge with oral, inhaled or nasal acetylsalicylic acid (ASA) is recommended for diagnostic purposes. Even if the clinical history is consistent, only 30% of patients have a positive response, while in children from a recent Spanish series, the response rate was 4%–10%.8 The placebo-controlled oral challenge test is considered the gold standard: increasing doses of ASA are administered, until the standard dose is reached. Approximate sensitivity of the test is 90%.1 FEV1 is recorded before each dose and 30min after, and the test is completed when FEV1 drops 20% or more from baseline.
The therapeutic approach involves the absolute avoidance of ASA/NSAIDs and drug treatment depending on the severity of the bronchial and nasal symptoms. Avoidance of COX-1 inhibitors is recommended; tolerance of selective COX-2 inhibitors is generally good, although some patients with poorly controlled asthma can also develop reactions.9
With regard to pharmacological management, these patients generally have moderate-severe symptoms and need to be treated in accordance with GEMA steps 4–5, with medium or high doses of inhaled corticosteroids with long-acting bronchodilators, combined in most cases with oral anti-leukotriene, which acts on the bronchial and nasal involvement.
When symptoms are refractory to these treatments, other alternatives can be evaluated, such as aspirin desensitization or biologics. Biologics include monoclonal anti-IgE (omalizumab is useful if IgE plays a priority role) and anti-IL5 antibodies, such as mepolizumab.
Mepolizumab is indicated in severe uncontrolled asthma with eosinophilia in plasma. Studies of mepolizumab in adults have demonstrated control of exacerbations, reduced eosinophils in blood, bone marrow and the airway, increased FEV1, reduced nasal polyps, and improved quality of life among patients.10,11 It is administered subcutaneously on a monthly basis, and the most common side effects are headache and local reaction at the injection site. Its use was authorized by the EMA in Europe in December 2015 for patients of 18 years of age and over, and by the FDA in the United States for patients older than 12 years.
It has been used in children in hypereosinophilic syndromes and eosinophilic esophagitis, and has shown persistent success after several years of treatment and a low rate of complications and side effects.12
In our hospital, we evaluated a 12-year-old boy with severe, poorly controlled asthma, who was admitted to the Pediatric ICU for pneumomediastinum associated with a respiratory infection and severe bronchospasm. He had no significant personal or family history, except for acute bronchiolitis at the age of 4 months, and later, 2 isolated episodes of wheezing in a context of viral infection, coinciding with starting school. He regularly practices sport, and is affiliated to the Football Federation. As environmental risk factors, it was noted that his mother smokes, but not at home, and the family has had a dog for about 6 years. Respiratory symptoms began 3 months before the above-mentioned admission, with clinical symptoms of dyspnea and wheezing, initially triggered by his sports activities. Similarly, he reported onset of mild and occasional rhinitis around 3–4 months previously, controlled with intranasal corticosteroids and oral antihistamines. Rhinitis had progressively worsened in recent months, with nasal obstruction and abundant rhinorrhea.
During the episode that prompted admission to the pediatric ICU and subsequent referral to the pediatric respiratory medicine department, the patient was treated with inhaled salbutamol, oral corticosteroids, and azithromycin. After that event, he started inhaled budesonide (Babyhaler spacer), at doses of 200μg every 12h. Progress was poor, and he had 6 moderate exacerbations in the next 3 months, so treatment was switched to salmeterol/fluticasone 50/100, one inhalation every 12h using a dry powder device. His drug administration technique was evaluated at all visits, and therapeutic compliance was assessed in the clinic by checking the use of inhalers, collection of medication from the pharmacy according to electronic prescribing records, and by interviewing the patient and his parents, who were informed that administration always had to be supervised. In the next visit, a poor application technique was detected, so the treatment was changed to inhalation using a spacer, at the same dose.
Additional tests included the skin prick test and a specific RAST that were negative for house dust mites, epithelia, molds, and pollens. Total IgE was slightly raised (325kU/l) and severe plasma eosinophilia was detected (24.3%). Baseline spirometry showed an obstructive pattern, with raised FeNO (maximum 108 ppb). Specifically, the first test showed a mild obstructive pattern, non-reversible after bronchodilator, and certain flattening of the inspiratory curve. In view of the atypical features of the case, a possible differential diagnosis of vocal cord dysfunction was proposed, and an exercise test was requested to try to demonstrate it. This test was positive for exercise-induced bronchospasm, with no involvement of the upper airways. The diagnostic possibility of vocal cord dysfunction was subsequently assessed urgently by the ENT department of the patient's hospital of origin during one of the exacerbations that led to admission, but this examination was normal.
In the following 2 months, the patient had another moderate exacerbation that required admission. He also had daily symptoms on minimal exertion, so the dose of inhaled corticosteroids was doubled to 25/250 in 2 inhalations every 12h. A 10-day cycle of oral corticosteroids was prescribed, with clinical and functional improvement during that period. It should be noted that one of the exacerbations that required admission during these months was associated with the administration of ibuprofen, with rapid improvement within less than 12h after admission.
In the following 2 months, he had another moderate-severe exacerbation requiring admission and another cycle of oral corticosteroids was administered, associated on this occasion with nasal and skin symptoms (facial and cervical erythema with subsequent urticarial rash). He continued to have symptoms, on minimal exertion during the day, and at night. He reported little symptomatic response to oral antihistamines and intranasal corticosteroids, but once again showed clinical and functional improvement after oral corticosteroids. For this reason, he was referred to the ENT department for evaluation of possible nasal polyposis. Grade II bilateral nasal polyps were confirmed on fiberoptic endoscopy.
Given the clinical suspicion of aspirin-exacerbated respiratory disease raised at this time, a challenge test was performed with oral ASA. After the third dose (44mg, accumulated dose 81mg), the patient developed sneezing, crystalline rhinorrhea, reddening of the skin, hyperventilation, wheezing on auscultation, and reduced FEV1 from a baseline FEV1 of 22%, so the test was considered positive.
In view of the confirmed diagnosis, the previous inhaled therapy with ICS + LABA was supplemented with oral montelukast 10mg, and strict avoidance of COX-1 inhibitors was prescribed.
Following this, he had no moderate or severe exacerbations and no need for oral corticosteroids, but always required terbutaline before practicing sport, and even so, had to rest and could only perform activities at a much less intensive level than his peers. He reported persistent daytime and night-time symptoms, and used his rescue bronchodilator as often as 3–4 times a day.
After receiving approval from the hospital and the boy's parents, we decided to start mepolizumab (anti-IL-5).
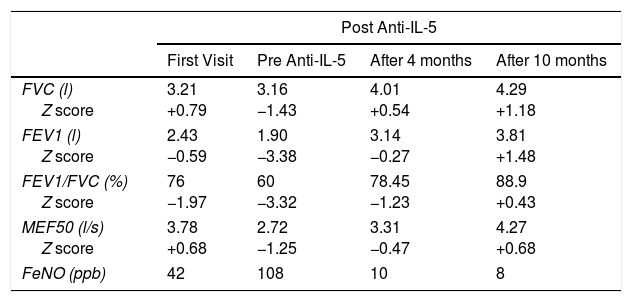
The patient has since shown significant clinical and functional improvement with good asthma control. He has not had any moderate exacerbations, and uses rescue bronchodilators only very occasionally. He has gradually taken up his sporting activity again, and has no problem performing intense exercise. His baseline spirometry has normalized (Table 1), and there has been a progressive reduction in the size of his nasal polyps, with the consequent improvement in symptoms. In view of this good progress, his ICS/LABA dose has been gradually reduced, and he is now receiving 1 inhalation of 50/100 every 12h using the dry powder device, after proper use of the technique was confirmed. He continues to receive 10mg montelukast every day and subcutaneous mepolizumab every 4 weeks. To date, no side effects have been observed, except for slight local edema at the injection site in the hours following administration of the drug.
Patient's Spirometry During Follow-up With Z-scores According to Age and Height.
| Post Anti-IL-5 | ||||
|---|---|---|---|---|
| First Visit | Pre Anti-IL-5 | After 4 months | After 10 months | |
| FVC (l) Z score | 3.21 +0.79 | 3.16 −1.43 | 4.01 +0.54 | 4.29 +1.18 |
| FEV1 (l) Z score | 2.43 −0.59 | 1.90 −3.38 | 3.14 −0.27 | 3.81 +1.48 |
| FEV1/FVC (%) Z score | 76 −1.97 | 60 −3.32 | 78.45 −1.23 | 88.9 +0.43 |
| MEF50 (l/s) Z score | 3.78 +0.68 | 2.72 −1.25 | 3.31 −0.47 | 4.27 +0.68 |
| FeNO (ppb) | 42 | 108 | 10 | 8 |
In conclusion, we report the case of a pediatric patient with an asthma phenotype more usually seen in adults. Poor response to escalation of his usual treatment led to the prescription of mepolizumab, and the patient has shown good clinical, laboratory and functional response 1 year after starting.
Please cite this article as: Méndez Sánchez A, Gutiérrez Martínez JR, Coca Pelaz A, Vázquez Piñera MA. Enfermedad respiratoria relacionada con aspirina tratada con mepolizumab en un paciente pediátrico. Arch Bronconeumol. 2019;55:55–57.