Nocardia is a strictly aerobic Gram-positive bacterium that is partially acid-alcohol-resistant and forms long branched filaments.1 Species of medical significance are: N. asteroides, N. brasiliensis, N. pseudobrasiliensis and N. otitidiscaviarum. Infection by the latter is rare compared with other species of Nocardia and rarely causes infection in humans, even in immunocompromised patients.2–4 All species are universally distributed and routinely found in soil, sand, dust, and stagnant water.2,3
We report a case of pyopneumothorax caused by Nocardia otitidiscaviarum, in an 89-year-old woman with mild persistent asthma, bronchiectasis in the left upper and lower lobes, and obesity. She had had 2 episodes of community-acquired pneumonia approximately 5 and 10 years previously. She had never smoked and could perform activities of daily living independently. Her usual medication consisted of benzodiazepines, proton pump inhibitor, and long-acting β-2 adrenergic bronchodilators combined with corticosteroids (budesonide/formoterol 160/4.5mcg, 1 inhalation every 12h) and on-demand short-acting β-2 adrenergic receptors for several years. She presented in the emergency department in December 2015 with a 1-week history of cough without expectoration, progressive dyspnea on moderate exertion, febrile sensation, constipation, nausea, and vomiting. She denied chest pain, dysuria, and other symptoms. On physical examination, she was conscious, oriented, and collaborative; she had tachypnea 20 breaths/minute, SpO2 86% breathing room air, blood pressure 133/55mmHg, heart rate 87 bpm, and axillary temperature 39.6°C. On pulmonary auscultation, breath sounds were normal, with abundant disperse rhonchi. The rest of the physical examination was normal. Chest X-ray was performed, showing right basal peribronchial thickening, cardiomegaly, and aortic elongation. ECG with no changes. Arterial blood gases: pH 7.46, pCO2 30mmHg, and pO2 48mmHg. Abnormal clinical laboratory results were sodium were 133mmol/l, LDH 460U/l, PCR 22mg/l, leukocytosis 10.83×109/l, and neutrophilia 9.35×109/l. Urine analysis showed moderate pyuria.
Diagnoses of acute partial respiratory failure, respiratory infection, and urinary infection were given, and the patient was admitted for antibiotic treatment in the form of levofloxacin, in addition to aerosols and corticosteroids. Escherichia coli in urine was confirmed on day 3 of admission.
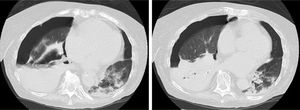
The patient's progress was poor. Eight days later, an image was observed in a radiographic follow-up of consolidation in the right middle and left lower lobes, so nosocomial pneumonia was suspected and antibiotic treatment was switched to ceftazidime and ciprofloxacin. On day 9, urine was positive for pneumococcal antigen. On days 12 and 16 after admission, N. otitidiscaviarum was isolated in a sputum culture, and treatment was changed, in line with the sensitivity studies, to cotrimoxazole, amikacin, and imipenem. In view of the worsening symptoms, a chest computed tomography was then performed (Fig. 1) which revealed right hydropneumothorax and areas of consolidation, some of which were cavitary, predominantly in the right lung base. A pleural drainage tube was placed, from which purulent fluid was obtained, determined to be predominantly polymorphonuclear exudate with a pH <7.2, indicating, more specifically, a case of pyopneumothorax. Throughout the period of admission, the patient continued to require high oxygen levels to maintain SatO2 at around 90%, and after suffering paralytic ileus that could not be corrected despite placement of a nasogastric and rectal tube, she died 35 days after admission.
Transversal CT slices of the chest performed on day 23 of admission, showing right hydropneumotorax, right lower lobe atelectasis and areas of consolidation, some cavitary, mainly in the right lung base. Cavitary pulmonary nodules and ground-glass opacities adjacent to the area of consolidation can be seen in the left lower lobe.
Nocardiosis is a suppurative infection that can occur acutely or chronically and tends to disseminate. It generally appears as an opportunistic infection in individuals with T cell-mediated immunosuppression. The most common risk factors are occult malignancies, diabetes, HIV, and COPD,3–5 although it can also occur in immunocompetent patients (up to one third of all cases).3,5,6 The most common routes of infection are inhalation and cutaneous inoculation.1,4,5 Pulmonary nocardiosis is the most common clinical presentation. This is a severe, sporadic disease that is more prevalent among men, and generally occurs in the fourth decade of life.2,7 Central nervous system and subcutaneous involvement may be present in up to 30% and 15% of cases, respectively.5,7
Risk factors for Nocardia infection in our patient were bronchiectasis and chronic use of inhaled corticosteroids. Radiological findings are variable and non-specific, and occur as consolidations, diffuse nodules or masses that may be cavitary, and less frequently, pleural effusion and diffuse interstitial patterns,4,6–11 although up to one-third of patients may not have radiological evidence of pulmonary involvement. This is a curable disease, but the mortality rate is high, and can be over 50% in severe cases with sudden onset.6,12 In patients with severe infection without central nervous system involvement, trimethoprim-sulfamethoxazole (15mg/kg/day intravenously), plus amikacin (7.5mg/kg/12h intravenously) is recommended.9 The initial treatment must be given intravenously for at least 3–6 months or until clinical improvement is documented. For severe pulmonary infection, the recommended treatment duration is 6–12 months or more.6,9,13
N. otitidiscaviarum tends to be resistant to trimethoprim-sulfamethoxazole, but it is usually sensitive to amikacin and minocycline.5,9 There are few reported cases of pyopneumotorax caused by Nocardia.8,12,14,15 Those cases that are reported were finally resolved, probably because the disease was diagnosed earlier and because it occurred in younger patients (aged 32, 47, 74 and 78 years). In our case, the patient died despite the administration of wide-spectrum antibiotic coverage, later adjusted to the sensitivity profile of the strain, perhaps because of her advanced age and late detection of complications.
Please cite this article as: Scheelje Carabelli T, Esteban Ronda V, Hernández Moreno K. Infección pulmonar por Nocardia otitidiscaviarum en paciente inmunocompetente. Arch Bronconeumol. 2019;55:59–60.